Abstract
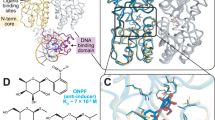
Comparison of the crystal structure of inactive unliganded trp aporepressor with that of trp repressor shows that binding tryptophan activates the dimer a thousandfold by moving two symmetrically-disposed flexible bihelical motifs. These flexible 'DNA-reading heads' flank a highly inflexible core domain formed by an unusual arrangement of interlocking α-helices from both subunits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Pabo, C. O. & Sauer, R. T. A. Rev. Biochem. 53, 293–321 (1984). 2. Dynan, W. S. & Tjian, R. Nature 316, 774–777 (1985). 3. Ptashne, M. A. Nature 321, 697–701 (1986). 4. Ptashne, M. A. A Genetic Switch PAGES? (Cell and Blackwell, Cambridge and Palo Alto, 1986). 5. Von Hippel, P. H., Bear, D. G., Morgan, W. D. & McSwiggen, J. A. A Rev. Biochem. 54, 389–446 (1984). 6. Crawford, I. P. & Stauffer, G. V. A. Rev. Biochem. 49, 163–195 (1980). 7. Platt, T. in The Operon, 2nd ed., (eds Miller, J. H., & Reznikoff, W. S.) 263–302 (Cold Spring Harbor Laboratory, New York, 1981). 8. Somerville, R. L. in Amino Acid Biosynthesis and Regulation (Herrmann, K. M. & Somerville, R. L.) 351–378 (Addison Westly, Massachusetts, 1983). 9. Joachimiak, A., Schevitz, R. W., Kelly, R. L., Yanofsky, C. & Sigler, P. B. J. biol. Chem. 258, 12641–12643 (1983). 10. Joachimiak, A. et al. J. biol Chem. 262, 4917–4921 (1987). 11. Bass, S., Sugiono, P., Arvidson, D. N., Gunsalus, R. P. & Youderian, P. Genes and Developmen t (submitted). 12. Schevitz, R. W., Otwinowski, Z., Joachimiak, A., Lawson, C. L. & Sigler, P. B. Nature 317, 782–786 (1985). 13. Hample, A. et al. Science 162, 1384–1387 (1968). 14. Schwert, G. W. R. & Kaufman, S. /. biol. Chem. 190, 807–816 (1951). 15. Joachimiak, A., Lelly, R. L., Gunsalus, R. P., Yanofsky, C. & Sigler, P. B. Proc. natn. Acad. Sci. U.S.A. 80, 668–672 (1983). 16. Rossmann, M. G. (ed.) The Molecular Replacement Method (Gordon and Breach, New York, 1972). 17. Crowther, R. A. in The Molecular Replacement Method (ed. Rossmann, M. G.) 173–178 (Gordon and Breach, New York, 1972). 18. Crowther, R. A. & Blow, D. Acta Cryst. 23, 544–549 (1967). 19. Sussman, J. L., Holbrook, S. R., Church, G. M. & Kirn, S. H. Acta Cryst A33, 800 (1977). 20. Hoard, L. G. & Nordman, C. E. Acta Cryst. A35, 1010 (1979). 21. Hendrickson, W. A. & Konnert, J. H. in Biomolecular Structure Function Conformation and Evolution (ed. Srinivasan, R.) 1, 43–57 (Pergamon, Oxford, 1980). 22. Jones, T. A. Meth. Enzym. (eds Wyckoff, H. W., Hirs, C. H. W. & Timasheff, S. N.) 115, PartB, 157–171 (Academic, Orlando, 1985). 23. Marmorstein, R. Q., Joachimiak, A., Sprinzl, M. & Sigler, P. B. /. biol. Chem. 262,4922–4927 (1987). 24. Arvidson, D. N., Bruce, C. & Gunsalus, R. P. /. biol Chem. 261, 238–243 (1986). 25. Lane, A. N. Eur. J. Biochem. (in the press). 26. Steitz, T. A., Ohlendorf, D. H., McKay, D. B., Anderson, W. F. & Matthews, B. W. Proc. natn. Acad. Sci. U.S.A. 79, 3097–3100 (1982). 27. Ohlendorf, D. H., Anderson, W. F. & Matthews, B. W. /. molec. Evol. 19, 109–114 (1983). 28. Kelly, R. L., Yanofsky, C. Proc. natn. Acad. Sci. U.S.A. 82, 483–487 (1985). 29. Pabo, C. O., Krovatin, W., Leffrey, A. & Sauer, R. T. Nature 298, 441–443 (1982). 30. Hoi, W. G. J., van Duijnen, P. T. & Berendsen, H. J. C. Nature 273, 443–446 (1978). 31. Berg, O. G., Winter, R. B. & Von Hippel, H. Biochem. 20, 6929–6948 (1981). 32. Sauer, R. T., Yocum, R. R., Doolittle, R. F., Lewis, M. & Pabo, C. O. Nature 298, 447–451 (1982). 33. Weber, I. T., McKay, D. B. & Steitz, T. A. Nucleic Acids Res. 10, 5085–5102 (1982). 34. Nishikawa, K., Ooi, T., Isogai, Y. & Saito, N. /. phys. Soc. Japan 32, 1331–1337 (1972). 35. Kundrot, C. E. & Richards, F. M. /. appl. Cryst. 19, 208–213 (1986). 36. Arnott, S. & Hukins, D. W. L. Biochem. Biophys. Res. Comm. 47, 1504–1509 (1972). 37. Gunsalus, R. P. & Yanofsky, C. Proc. natn. Acad. Sci. U.S.A. 77, 7117–7121 (1980). 38. Ohlendorf, D. H., Anderson, W. F., Takeda, Y. & Matthews, B. W. /. Biomolec. Struct. Dynamics 1, 553–563 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, Rg., Joachimiak, A., Lawson, C. et al. The crystal structure of trp aporepressor at 1.8 Å shows how binding tryptophan enhances DNA affinity. Nature 327, 591–597 (1987). https://doi.org/10.1038/327591a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/327591a0
This article is cited by
-
A biosensor for the direct visualization of auxin
Nature (2021)
-
Directed evolution of a synthetic phylogeny of programmable Trp repressors
Nature Chemical Biology (2018)
-
Effect of mutation at the interface of Trp-repressor dimeric protein: a steered molecular dynamics simulation
European Biophysics Journal (2013)
-
Deciphering the transcriptional regulatory logic of amino acid metabolism
Nature Chemical Biology (2012)
-
Probing the relation between protein structure and intrinsic tryptophan fluorescence using superrepressor mutants of thetrp repressor
Journal of Fluorescence (1998)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.