Abstract
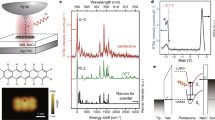
BOHR'S notion of quantum jumps between electronic states of an excited atom has now been demonstrated experimentally for single ions confined in radio-frequency traps and interacting with a driving laser field1–3. In these experiments the fluorescence of a strongly allowed transition was shown to cease abruptly when the ion jumped into a metastable state which was coupled to the common electronic ground state by a weak radiative transition. But attempts to monitor quantum jumps of single molecules have been hampered by the fact that the lifetime of the metastable triplet state was too short in relation to the photon detection rate. By using a system with favourable photophysical parameters—terrylene doped into p-terphenyl crystals4—we have now been able to observe directly quantum jumps between electronic states of single terrylene molecules. In contrast to single atoms, here the quantum jumps occur as non-radiative transitions between states of different multiplicity, and are manifested as interruptions of the fluorescence signal. These results demonstrate how single-molecule spectros-copy can reveal truly quantum-mechanical effects in large polyatomic molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nagourney, W., Sandberg, J. & Dehmelt, H. Phys. Rev. Lett. 56, 2797–2799 (1986).
Sauter, Th., Neuhauser, W., Blatt, R. & Toschek, P. E. Phys. Rev. Lett. 57, 1696–1698 (1986).
Bergquist, J. C., Hulet, R. G., Itano, W. M. & Wineland, D. J. Phys. Rev. Lett. 57, 1699–1702 (1986).
Kummer, S., Basché, Th. & Bräuchle, C. Chem. Phys. Lett. 229, 309–316 (1994).
Ambrose, W. P., Basché, Th. & Moerner, W. E. J. chem. Phys. 95, 7150–7163 (1991).
Moerner, W. E. & Basché, Th. Angew. chem. 105, 537–557 (1993); Angew. Chem. int. Edn engl. 32, 457–476 (1993).
Schenzle, A. & Brewer, R. G. Phys. Rev. A34, 3127–3142 (1986).
Birks, J. B. Photophysics of Aromatic Molecules (Wiley-lnterscience, London, 1970).
Bernard, J., Fleury, L., Talon, H. & Orrit, M. J. chem. Phys. 98, 850–859 (1993).
Basché, Th., Moerner, W. E., Orrit, M. & Talon, H. Phys. Rev. Lett. 69, 1516–1519 (1992).
McGlynn, S. P., Azumi, T. & Kinoshita, M. Molecular Spectroscopy of the Triplet State (Prentice Hall, Englewood Cliffs, 1969).
Lawetz, V., Orlandi, G. & Siebrand, W. J. chem. Phys. 56, 4058–4072 (1972).
Köhler, J. et al. Nature 363, 242–244 (1993).
Wrachtrup, J., von Borczyskowski, C., Bernard, J., Orrit, M. & Brown, R. Nature 363, 244–245 (1993).
Cook, R. J. & Kimble, H. J. Phys. Rev. Lett. 54, 1023–1026 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Basché, T., Kummer, S. & Bräuchle, C. Direct spectroscopic observation of quantum jumps of a single molecule. Nature 373, 132–134 (1995). https://doi.org/10.1038/373132a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/373132a0
This article is cited by
-
Sharp zero-phonon lines of single organic molecules on a hexagonal boron-nitride surface
Nature Communications (2023)
-
To catch and reverse a quantum jump mid-flight
Nature (2019)
-
Observation of spin-dependent quantum jumps via quantum dot resonance fluorescence
Nature (2010)
-
Quantum jumps of light recording the birth and death of a photon in a cavity
Nature (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.