Abstract
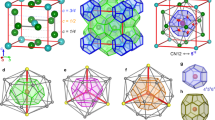
Interactions between hydrophobic groups in water1, as well as biomolecular hydration more generally2,3,4, are intimately connected to the structure of liquid water around hydrophobic solutes. Such considerations have focused interest on clathrate hydrates: crystals in which a hydrogen-bonded network of watermolecules encages hydrophobic guest molecules with which the water interacts only by non-directional van der Waals forces. Three structural families of clathrate hydrates have hitherto been recognized: cubic structure I (2MS·6ML·46H2O) (ref. 5), cubic structure II (16MS·8ML·136H2O) (ref. 5) and hexagonal structure H (ML·3MS·2MS·34H2O) (refs 6, 7) hydrates (here ML and MS are the hydrophobic guest sites associated with large and small cavities, respectively). Here we report a new hydrate structure: 1.67 choline hydroxide·tetra- n-propylammonium fluoride·30.33H2O. This structure has a number of unusual features; in particular the choline guest exhibits both hydrophobic and hydrophilic modes of hydration. Formally the structure consists of alternating stacks of structure H and structure II hydrates, and might conceivably be found in those settings (such as seafloor deposits over natural-gas fields) in which clathrate hydrates form naturally.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blokzyl, W. & Engberts, J. B. F. Hydrophobic effects. Opinions and facts. Angew. Chem. Int. Edn Engl. 32, 1545–1579 (1993).
Head-Gordon, T. Is water structure around hydrophobic groups clathrate-like? Proc. Natl Acad. Sci. USA 92, 8308–8312 (1995).
Lipscomb, L. A., Zhou, F. X. & Williams, L. D. Clathrate hydrates are poor models of biomolecule hydration. Biopolymers 38, 177– 181 (1996).
Bouquiere, J. P., Finney, J. L. & Savage, H. F. J. High-resolution neutron study of vitamin B12 coenzyme at 15K: solvent structure. Acta Crystallogr. B 50, 566–578 (1994).
Jeffrey, G. A. in Inclusion Compounds Vol. 1(eds Atwood, J. L, Davies, J. E. D. & MacNicol, D. D.) 135–190 (Academic, London, (1984)).
Ripmeester, J. A., Ratcliffe, C. I., Tse, J. S. & Powell, B. M. Anew clathrate hydrate structure. Nature 325, 135–136 (1987).
Udachin, K. A., Ratcliffe, C. I., Enright, G. D. & Ripmeester, J. A. Structure H hydrate: a single crystal diffraction study of 2,2 dimethylpentane.5(Xe,H 2S).34H2O. Supramol. Chem. 8, 173–176 (1997).
Lipkowski, J., Luboradzki, R., Udachin, K. A. & Dyadin, Yu. A. Alayered hydrate structure of tetrapropyl ammonium fluoride. J. Inclus. Phenom. 13, 295 (1992 ).
Dyadin, Yu. A., Udachin, K. A., Zhurko, F. V. & Mironov, Yu. I. Cubic structure II double clathrate hydrates with tetra-n-propylammonium fluoride. J. Inclus. Phenom. 6, 565– 575 (1988).
Martorana, V., Bulone, D., San Biagio, P. L., Palma-Vitorelli, M. B. & Palma, M. U. Collective properties of hydration—long range and specificity of hydrophobic interactions. Biophys. J. 73, 31–37 (1997).
Smelik, E. A. & King, H. E. J Clathrate hydrates as layer structures. Comparison between the structures of type II and hypothetical type V. Z. Kristallogr. 211, 84–89 (1996).
Gies, H. in Inclusion Compounds Vol. 5(eds Atwood, J. L., Davies, J. E. D. & MacNicol, D. D.) 1–31 (Oxford Univ. Press, (1991)).
Davidson, D. W. et al. Laboratory analysis of a naturally occurring gas hydrate. Geochim. Cosmochim. Acta 50, 619– 623 (1986).
Sassen, R. & MacDonald, I. R. Evidence of structure H hydrate. Gulf of Mexico continental shelf. Org. Geochem. 22, 1029–1032 (1994).
Kvenvolden, K. A. Natural gas hydrate occurrence and issues. Ann. N.Y. Acad. Sci. 715, 232–247 ( 1994).
Sloan, E. D. J Clathrate Hydrates of Natural Gas 2nd edn (Dekker, New York, ( 1996)).
Sheldrick, G. M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. A 46 467–473 (1990).
Sheldrick, G. M. Anew-least squares refinement program for use with single crystal diffraction data. Acta Crystallogr. A 49 (Suppl.), C53 C53 (1993).
Acknowledgements
We thank the NATO Science Programme for partial support of this work in the form of a Collaborative Research Grant to K.A.U.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Udachin, K., Ripmeester, J. A complex clathrate hydrate structure showing bimodal guest hydration . Nature 397, 420–423 (1999). https://doi.org/10.1038/17097
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/17097
This article is cited by
-
Influence of temperature on methane hydrate formation
Scientific Reports (2017)
-
Self-preservation and structural transition of gas hydrates during dissociation below the ice point: an in situ study using Raman spectroscopy
Scientific Reports (2016)
-
Synthesis, Crystal Structure and Thermal Property of a Novel Mn(II) Coordination Polymer Bridged by Imazapyr
Journal of Chemical Crystallography (2011)
-
Synthesis, spectral, and thermal properties, and crystal structure of cobalt (III) complex derived from Imazameth
Structural Chemistry (2010)
-
A spectroscopic method to estimate the binding potency of amphiphile assemblies
Analytical and Bioanalytical Chemistry (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.