Abstract
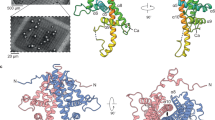
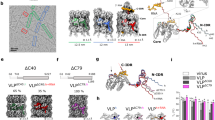
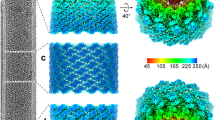
Cypoviruses and baculoviruses are notoriously difficult to eradicate because the virus particles are embedded in micrometre-sized protein crystals called polyhedra1,2. The remarkable stability of polyhedra means that, like bacterial spores, these insect viruses remain infectious for years in soil. The environmental persistence of polyhedra is the cause of significant losses in silkworm cocoon harvests but has also been exploited against pests in biological alternatives to chemical insecticides3,4. Although polyhedra have been extensively characterized since the early 1900s5, their atomic organization remains elusive6. Here we describe the 2 Å crystal structure of both recombinant and infectious silkworm cypovirus polyhedra determined using crystals 5–12 micrometres in diameter purified from insect cells. These are the smallest crystals yet used for de novo X-ray protein structure determination7. We found that polyhedra are made of trimers of the viral polyhedrin protein and contain nucleotides. Although the shape of these building blocks is reminiscent of some capsid trimers, polyhedrin has a new fold and has evolved to assemble in vivo into three-dimensional cubic crystals rather than icosahedral shells. The polyhedrin trimers are extensively cross-linked in polyhedra by non-covalent interactions and pack with an exquisite molecular complementarity similar to that of antigen–antibody complexes. The resulting ultrastable and sealed crystals shield the virus particles from environmental damage. The structure suggests that polyhedra can serve as the basis for the development of robust and versatile nanoparticles for biotechnological applications8 such as microarrays9 and biopesticides4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Belloncik, S. & Mori, H. in The Insect Viruses (eds Miller, L. K. & Ball, L. A.) 337–369 (Plenum, New York, 1998)
Miller, L. K. The Baculoviruses (Plenum, New York, 1997)
Summers, M. D. Milestones leading to the genetic engineering of baculoviruses as expression vector systems and viral pesticides. In Advances in Virus Research (eds Bonning, B. C., Maramorosch, K. & Shatkin, A. J.), Ch. 68 3–73 (Academic Press, New York, 2006)
Chang, J. H. et al. An improved baculovirus insecticide producing occlusion bodies that contain Bacillus thuringiensis insect toxin. J. Invertebr. Pathol. 84, 30–37 (2003)
Glaser, R. W. & Chapman, J. W. The nature of the polyhedral bodies found in insect cells. Biol. Bull. 30, 367–390 (1916)
Rohrmann, G. F. Polyhedrin structure. J. Gen. Virol. 67, 1499–1513 (1986)
Riekel, C., Burghammer, M. & Schertler, G. Protein crystallography microdiffraction. Curr. Opin. Struct. Biol. 15, 556–562 (2005)
Douglas, T. & Young, M. Viruses: making friends with old foes. Science 312, 873–875 (2006)
Ikeda, K. et al. Immobilization of diverse foreign proteins in viral polyhedra and potential application for protein microarrays. Proteomics 6, 54–66 (2006)
Fields, B. N., Knipe, D. M., Howley, P. M. & Griffin, D. E. Field's Virology (Lippincott Williams & Wilkins, Philadelphia, 2001)
Zhang, H. et al. Visualization of protein-RNA interactions in cytoplasmic polyhedrosis virus. J. Virol. 73, 1624–1629 (1999)
Hill, C. L. et al. The structure of a cypovirus and the functional organization of dsRNA viruses. Nature Struct. Mol. Biol. 6, 565–568 (1999)
Thorne, R. E., Stum, Z., Kmetko, J., O'Neill, K. & Gillilan, R. Microfabricated mounts for high-throughput macromolecular cryocrystallography. J. Appl. Cryst. 36, 1455–1460 (2003)
Doye, J. P. K. & Poon, W. C. K. Protein crystallization in vivo. Curr. Opin. Colloid Interf. Sci. 11, 40–46 (2006)
Grimes, J., Basak, A. K., Roy, P. & Stuart, D. The crystal structure of bluetongue virus VP7. Nature 373, 167–170 (1995)
Liemann, S., Chandran, K., Baker, T. S., Nibert, M. L. & Harrison, S. C. Structure of the reovirus membrane-penetration protein, Mu1, in a complex with its protector protein, Sigma3. Cell 108, 283–295 (2002)
Coulibaly, F. et al. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 120, 761–772 (2005)
Wodak, S. J. & Janin, J. Structural basis of macromolecular recognition. Adv. Protein Chem. 61, 9–73 (2002)
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993)
Fujiwara, T. et al. Xray diffraction studies of the polyhedral inclusion bodies of nuclear and cytoplasmic polyhedrosis viruses. Appl. Entomol. Zool. Jpn 19, 402–403 (1984)
Anduleit, K. et al. Crystal lattice as biological phenotype for insect viruses. Protein Sci. 14, 2741–2743 (2005)
Mori, H. et al. Expression of Bombyx mori cytoplasmic polyhedrosis virus polyhedrin in insect cells by using a baculovirus expression vector, and its assembly into polyhedra. J. Gen. Virol. 74, 99–102 (1993)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology (eds Carter, C. W. & Sweet, R. M.) Ch. 276 307–326 (Academic Press, New York, 1997)
Schneider, T. R. & Sheldrick, G. M. Substructure solution with SHELXD. Acta Crystallogr. D 58, 1772–1779 (2002)
de La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. In Methods in Enzymology (eds Carter, C. W. & Sweet, R. M.) Ch. 276 472–494 (Academic Press, New York, 1997)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Lovell, S. C. et al. Structure validation by Cα geometry: φ, ψ and Cβ deviation. Proteins 50, 437–450 (2003)
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Krissinel, E. & Henrick, K. Detection of protein-assemblies in crystals. In Lecture Notes in Computer Science (ed. Berthold, M. R.) Ch. 3695 163–174 (Springer, Berlin/Heidelberg, 2005)
Kleywegt, G. J. & Jones, T. A. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. D 50, 178–185 (1994)
Acknowledgements
We are very grateful for support from E. N. Baker. We thank R. E. Thorne for providing MicroMesh mounts; J. Taylor, A. Mitra and colleagues at SBS for critically reading the manuscript; and M. Middleditch, C. Hobbis, R. Graves, R. Bunker, A. Wagner, E. Pohl and J. Diez for experimental help. S.G. is funded by the Swiss NCCR Structural Biology and E.C. by a TEAC Bright Futures doctoral scholarship. This work was supported by the NZ FRST (F.C.), the UARC (P.M.), the Maurice Wilkins Centre for Molecular Biodiscovery (E.C.) and grants for Regional Consortium Research Development Work from the Kansai Bureau of METI (K.I. and H.M.), from CREST by JST (H.M.) and a JSPS Invitation Fellowship for Research in Japan (P.M.).
Structures of polyhedrins from infectious, recombinant and kinase-containing polyhedra have been deposited in the Protein Data Bank with accession codes 2OH5, 2OH6 and 2OH7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S3 with Legends. (PDF 2816 kb)
Rights and permissions
About this article
Cite this article
Coulibaly, F., Chiu, E., Ikeda, K. et al. The molecular organization of cypovirus polyhedra. Nature 446, 97–101 (2007). https://doi.org/10.1038/nature05628
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05628
This article is cited by
-
Atomic structure of a nudivirus occlusion body protein determined from a 70-year-old crystal sample
Nature Communications (2023)
-
Tight junction protein claudin-2 promotes cell entry of Bombyx mori cypovirus
Applied Microbiology and Biotechnology (2021)
-
Construction of gateway-compatible baculovirus expression vectors for high-throughput protein expression and in vivo microcrystal screening
Scientific Reports (2020)
-
In cellulo crystallization of Trypanosoma brucei IMP dehydrogenase enables the identification of genuine co-factors
Nature Communications (2020)
-
Cell-line-dependent crystal morphology and sublocalization of the Thyrinteina arnobia cypovirus polyhedrin expressed from a recombinant baculovirus
Archives of Virology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.