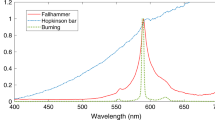
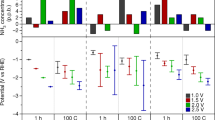
Abstract
IT has been appreciated for many years1–5 that ammonium nitrate, even when free from combustible material, can detonate. This is not surprising, since the products of decomposition must be entirely gaseous and have substantially less energy of formation than the original substance. For example, if the nitrogen is not oxidized, the energy released at constant volume, with water in the vapour phase, is 380 cal./gm. or 8.7 kcal. per mole of gas. This is, of course, rather low compared with typical values for explosives, which lie in the range 20–50 kcal./mole gas. The temperatures and pressures reached in the detonation wave will therefore also be relatively low, and one might expect the substance to detonate with difficulty and only under good confinement. This is found to be so. For normal material at densities around 1 gm./cm.3, cartridge diameters of many inches or heavy metal tubes are necessary6,7, and even then the measured speeds are much lower than the maximum values calculated for products N2 + 2H2O + ½O2. This has led eome authors8 to conclude that an equilibrium involving oxides of nitrogen is more appropriate, and that the release of energy is of order 230 cal./gm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Munroe, C. E., Chem. Met. Eng., 26, 535 (1922).
Aufschläger, R., Z. ges. Schiess.- und Spreng., 18, 117 (1923).
Gawthrop, D. B., Army Ordn., 6, 47 (1925).
Kast, H., Z. ges. Schiess.- und Spreng., 21, 205 (1926); 22, 6 (1927).
Later references: Lewis, B., U.S. Bur. Mines R.I. 4502 (1949).
Taylor, J., Detonation in Condensed Explosives (Oxford Univ. Press, 1952).
Cook, M. A., Mayfield, E. B., and Partridge, W. S., J. Phys. Chem., 59, 675 (1955).
Ide, K. H., Haeuseler, E., and Swart, K.-H., Explosifstoffe, 9, 195 (1961).
Fukuyama, I., J. Indust. Expl. Soc. Japan, 20, 119 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PATERSON, S., DAVIDSON, J. Detonation in Ammonium Nitrate. Nature 195, 277–278 (1962). https://doi.org/10.1038/195277a0
Issue Date:
DOI: https://doi.org/10.1038/195277a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.