Abstract
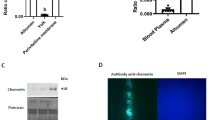
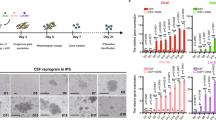
The tubular gland of the chicken oviduct is an attractive system for protein expression as large quantities of proteins are deposited in the egg, the production of eggs is easily scalable and good manufacturing practices for therapeutics from eggs have been established. Here we examined the ability of upstream and downstream DNA sequences of ovalbumin, a protein produced exclusively in very high quantities in chicken egg white, to drive tissue-specific expression of human mAb in chicken eggs. To accommodate these large regulatory regions, we established and transfected lines of chicken embryonic stem (cES) cells and formed chimeras that express mAb from cES cell–derived tubular gland cells. Eggs from high-grade chimeras contained up to 3 mg of mAb that possesses enhanced antibody-dependent cellular cytotoxicity (ADCC), nonantigenic glycosylation, acceptable half-life, excellent antigen recognition and good rates of internalization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ma, J.K., Drake, P.M. & Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 4, 794–805 (2003).
Ward, M. et al. Characterization of humanized antibodies secreted by Aspergillus niger. Appl. Environ. Microbiol. 70, 2567–2576 (2004).
Echelard, Y. Recombinant protein production in transgenic animals. Curr. Opin. Biotechnol. 7, 536–540 (1996).
Edmunds, T. et al. Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood 91, 4561–4571 (1998).
Pollock, D.P. et al. Transgenic milk as a method for the production of recombinant antibodies. J. Immunol. Methods 231, 147–157 (1999).
Kaye, J.S., Bellard, M., Dretzen, G., Bellard, F. & Chambon, P. A close association between sites of DNase I hypersensitivity and sites of enhanced cleavage by micrococcal nuclease in the 5′-flanking region of the actively transcribed ovalbumin gene. EMBO J. 3, 1137–1144 (1984).
Kaye, J.S. et al. Steroid hormone dependence of four DNase I-hypersensitive regions located within the 7000-bp 5′-flanking segment of the ovalbumin gene. EMBO J. 5, 277–285 (1986).
Kato, S. et al. A far upstream estrogen response element of the ovalbumin gene contains several half-palindromic 5′-TGACC-3′ motifs acting synergistically. Cell 68, 731–742 (1992).
Ghirlando, R., Lund, J., Goodall, M. & Jefferis, R. Glycosylation of human IgG-Fc: influences on structure revealed by differential scanning micro-calorimetry. Immunol. Lett. 68, 47–52 (1999).
Liu, H. et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 58, 4055–4060 (1998).
Bosselman, R.A. et al. Germline transmission of exogenous genes in the chicken. Science 243, 533–535 (1989).
Cook, R.F. et al. Liver-specific expression of a phosphoenolpyruvate carboxykinase-neo gene in genetically modified chickens. Poult. Sci. 72, 554–567 (1993).
Hippenmeyer, P.J., Krivi, G.G. & Highkin, M.K. Transfer and expression of the bacterial NPT-II gene in chick embryos using a Schmidt-Ruppin retrovirus vector. Nucleic Acids Res. 16, 7619–7632 (1988).
Harvey, A.J., Speksnijder, G., Baugh, L.R., Morris, J.A. & Ivariet, R. Consistent production of transgenic chickens using replication-deficient retroviral vectors and high-throughput screening procedures. Poult. Sci. 81, 202–212 (2002).
Harvey, A.J., Speksnijder, G., Baugh, L.R., Morris, J.A. & Ivarie, R. Expression of exogenous protein in the egg white of transgenic chickens. Nat. Biotechnol. 20, 396–399 (2002).
Rapp, J.C., Harvey, A.J., Speksnijder, G.L., Hu, W. & Ivarie, R. Biologically active human interferon alpha-2b produced in the egg white of transgenic hens. Transgenic Res. 12, 569–575 (2003).
McGrew, M.J. et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 5, 728–733 (2004).
Pain, B. et al. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development 122, 2339–2348 (1996).
Petitte, J.N., Liu, G. & Yang, Z. Avian pluripotent stem cells. Mech. Dev. 121, 1159–1168 (2004).
Acloque, H. et al. Identification of a new gene family specifically expressed in chicken embryonic stem cells and early embryo. Mech. Dev. 103, 79–91 (2001).
Shields, R.L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Okazaki, A. et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J. Mol. Biol. 336, 1239–1249 (2004).
Niwa, R. et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 64, 2127–2133 (2004).
Raju, T.S., Briggs, J.B., Borge, S.M. & Jones, A.J.S. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10, 477–486 (2000).
Suzuki, N., Khoo, K.H., Chen, C.M., Chen, H.C. & Lee, Y.C. N-glycan structures of pigeon IgG: a major serum glycoprotein containing Galalpha1–4 Gal termini. J. Biol. Chem. 278, 46293–46306 (2003).
Iwase, H., Kato, Y., Li, S.C., Li, Y.T. & Hotta, K. Comparative study of avian ovalbumins by means of glycosidase treatment and following HPLC analysis of their dansyl glycopeptides. Comp. Biochem. Physiol. B 79, 321–324 (1984).
Hase, S., Sugimoto, T., Takemoto, H., Ikenaka, T. & Schmid, K. The structure of sugar chains of Japanese quail ovomucoid. The occurrence of oligosaccharides not expected from the classical biosynthetic pathway for N-glycans; a method for the assessment of the structure of glycans present in picomolar amounts. J. Biochem. 99, 1725–1733 (1986).
Takahashi, N. et al. A structural study of the asparagine-linked oligosaccharide moiety of duck ovomucoid. Glycoconj. J. 10, 425–434 (1993).
Kuster, B., Naven, T.J. & Harvey, D.J. Rapid approach for sequencing neutral oligosaccharides by exoglycosidase digestion and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Mass Spectrom. 31, 1131–1140 (1996).
Harvey, D.J., Wing, D.R., Kuster, B. & Wilson, I.B. Composition of N-linked carbohydrates from ovalbumin and co-purified glycoproteins. J. Am. Soc. Mass Spectrom. 11, 564–571 (2000).
Takahashi, N., Khoo, K.H., Suzuki, N., Johnson, J.R. & Lee, Y.C. N-glycan structures from the major glycoproteins of pigeon egg white: predominance of terminal Galalpha(1)Gal. J. Biol. Chem. 276, 23230–23239 (2001).
Saba, J.A., Shen, X., Jamieson, J.C. & Perreault, H. Investigation of different combinations of derivatization, separation methods and electrospray ionization mass spectrometry for standard oligosaccharides and glycans from ovalbumin. J. Mass Spectrom. 36, 563–574 (2001).
Clynes, R.A., Towers, T.L., Presta, L.G. & Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Niwa, R. et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin. Cancer Res. 10, 6248–6255 (2004).
Ivarie, R. Avian transgenesis: progress towards the promise. Trends Biotechnol. 21, 14–19 (2003).
Lillico, S.G., McGrew, M.J., Sherman, A. & Sang, H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today 10, 191–196 (2005).
Eyal-Giladi, H. & Kochav, S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick I. General Morphology. Dev. Biol. 49, 321–337 (1976).
Petitte, J.N. in Handbook of Stem Cells vol 1. (ed. Lanza, R.P.) 471–477 (Elsevier Academic, London, 2004).
Rowlett, K. & Simkiss, K. Explanted embryo culture: in vitro and in ovo techniques for domestic fowl. Br. Poult. Sci. 28, 91–101 (1987).
Carsience, R.S., Clark, M.E., Verrinder Gibbins, A.M. & Etches, R.J. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development 117, 669–675 (1993).
Crooijmans, R.P., Vrebalov, J., Dijkhof, R.J.M., van der Poel, J.J. & Groenen, M.A.M. Two-dimensional screening of the Wageningen chicken BAC library. Mamm. Genome 11, 360–363 (2000).
Niwa, H., Yamamura, K.-I. & Miyazaki, J.-I. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–200 (1991).
Dangl, J.L. et al. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 7, 1989–1994 (1988).
Fishwild, D. et al. High-avidity human IgGk monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 14, 845–851 (1996).
Holmes, E.H. PMSA specific antibodies and their diagnostic and therapeutic use. Expert Opin. Investig. Drugs 10, 511–519 (2001).
Mohammed, S.M. et al. Deposition of genetically engineered human antibodies into the egg yolk of hens. Immunotechnology 4, 115–125 (1998).
Kodama, H. et al. Nucleotide sequences and unusual electrophoretic behavior of the W chromosome-specific repeating DNA units of the domestic fowl, Gallus gallus domesticus. Chromosoma 96, 18–25 (1987).
Kato, A., Nakamura, R. & Yasushi, S. Changes in the chemical composition of ovomucin during storage. Agric. Biol. Chem. 34, 1009–1013 (1970).
Acknowledgements
The work conducted at Origen Therapeutics and Texas A&M University was supported by the National Institutes of Health Small Business Innovation Research Grant GM064261. Research in the laboratory at UCLA was supported by grants AI29470, AI39187 and AI51415 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Many of the authors are employees of companies developing these technologies for commerical application.
Supplementary information
Supplementary Fig. 1
Southern blot analysis of cES clones transfected with Ov7.5 and Ov15 expression vectors. (PDF 1575 kb)
Supplementary Fig. 2
The morphology of the female chicken embryonic stem (ES) cell line 440 is shown in the upper panel. (PDF 125 kb)
Supplementary Fig. 3
cIEF data for MAbF1 produced by chicken tubular gland cells and CHO cells. (PDF 377 kb)
Supplementary Fig. 4
Thermal stabilities of chicken and CHO cell derived MAbF1. (PDF 1893 kb)
Supplementary Table 1
The extent of feather chimerism and the number of offspring obtained from male chimeras carrying cES cells derived from Barred Plymouth Rock. (PDF 31 kb)
Supplementary Table 2
Sequences for PCR primers. (PDF 37 kb)
Supplementary Table 3
Sequence analysis of chicken produced and CHO produced MAbF1 by mass spectrometry. (PDF 50 kb)
Rights and permissions
About this article
Cite this article
Zhu, L., van de Lavoir, MC., Albanese, J. et al. Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol 23, 1159–1169 (2005). https://doi.org/10.1038/nbt1132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1132
This article is cited by
-
Study of the regulatory elements of the Ovalbumin gene promoter using CRISPR technology in chicken cells
Journal of Biological Engineering (2023)
-
Production of recombinant human IgG1 Fc with beneficial N-glycosylation pattern for anti-inflammatory activity using genome-edited chickens
Communications Biology (2023)
-
Delivery of Fc-fusion Protein by a Recombinant Newcastle Disease Virus Vector
Applied Biochemistry and Biotechnology (2023)
-
CRISPR/Cas9 gene editing in a chicken model: current approaches and applications
Journal of Applied Genetics (2020)
-
Retinoic acid (RA) and bone morphogenetic protein 4 (BMP4) restore the germline competence of in vitro cultured chicken blastodermal cells
In Vitro Cellular & Developmental Biology - Animal (2019)