Abstract
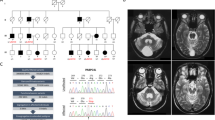
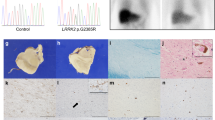
Hallervorden-Spatz syndrome (HSS) is an autosomal recessive neurodegenerative disorder associated with iron accumulation in the brain. Clinical features include extrapyramidal dysfunction, onset in childhood, and a relentlessly progressive course1. Histologic study reveals iron deposits in the basal ganglia2. In this respect, HSS may serve as a model for complex neurodegenerative diseases, such as Parkinson disease3, Alzheimer disease4, Huntington disease5 and human immunodeficiency virus (HIV) encephalopathy6, in which pathologic accumulation of iron in the brain is also observed. Thus, understanding the biochemical defect in HSS may provide key insights into the regulation of iron metabolism and its perturbation in this and other neurodegenerative diseases. Here we show that HSS is caused by a defect in a novel pantothenate kinase gene and propose a mechanism for oxidative stress in the pathophysiology of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dooling, E.C., Schoene, W.C. & Richardson, E.P. Jr. Hallervorden-Spatz syndrome. Arch. Neurol. 30, 70–83 (1974).
Swaiman, K.F. Hallervorden-Spatz syndrome and brain iron metabolism. Arch. Neurol. 48, 1285–1293 (1991).
Sofic, E.,. et al. Increased iron III and total iron content in postmortem substantia nigra in parkinsonian brain. J. Neural Trans. 74, 199–205 (1988).
Connor, J.R., Snyder, B.S., Beard, J.L., Fine, R.E. & Mufson, E.J. Regional distribution of iron and iron regulatory proteins in the brain in aging and Alzheimer's disease. J. Neurosci. Res. 31, 327–335 (1992).
Dexter, D.T.,. et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting basal ganglia. Brain 114, 1953–1975 (1991).
Miszkziel, K.A.,. et al. The measurement of R-2, R-2-* and R-2′ in HIV-infected patients using the prime sequence as a measure of brain iron deposition. Magn. Reson. Imaging 15, 1113–1119 (1997).
Angelini, L.,. et al. Hallervorden-Spatz disease: clinical and MRI study of 11 cases diagnosed in life. J. Neurol. 239, 417–425 (1992).
Taylor, T.D.,. et al. Homozygosity mapping of Hallervorden-Spatz syndrome to chromosome 20p12.3–p13. Nature Genet. 14, 479–481 (1996).
Rock, C.O., Calder, R.B., Karim, M.A. & Jackowski, S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J. Biol. Chem. 275, 1377–1383 (2000).
Coppeto, J.R. & Lessell, S. A familial syndrome of dystonia, blepharospasm, and pigmentary retinopathy. Neurology 40, 1359–1363 (1990).
Gravel, R.A.,. et al. Mutations participating in interallelic complementation in propionic acidemia. Am. J. Hum. Genet. 55, 51–58 (1994).
Yun, M.,. et al. Structural basis for the feedback regulation of Escherichia coli pantothenate kinase by coenzyme A. J. Biol. Chem. 275, 28093–28099 (2000).
Blackwood, E.M.,. et al. Functional analysis of the AUG- and CUG-initiated forms of the c-Myc protein. Mol. Biol. Cell 5, 597–609 (1994).
Kozak, M. The scanning model for translation: an update. J. Cell Biol. 108, 229–241 (1989).
Kozak, M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA 87, 8301–8305 (1990).
Afshar, K., Gonczy, P., DiNardo, S. & Wasserman, S.A. fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics 157, 1267–1276 (2001).
Karim, M.A., Valentine, V.A. & Jackowski, S. Human pantothenate kinase 1 ( PANK1 ) gene: Characterization of the cDNAs, structural organization and mapping of the locus to chromosome 10q23.2-23.31. Am. J. Hum. Genet. 67, A984 (2000).
Abiko, Y. Investigations on pantothenic acid and its related compounds. IX. Biochemical studies. 4. Separation and substrate specificity of pantothenate kinase and phosphopantothenoylcysteine synthetase. J. Biochem. (Tokyo) 61, 290–299 (1967).
Winzeler, E.A.,. et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 (1999).
Calder, R.B.,. et al. Cloning and characterization of a eukaryotic pantothenate kinase gene (panK) from Aspergillus nidulans. J. Biol. Chem. 274, 2014–2020 (1999).
Vallari, D.S. & Rock, C.O. Isolation and characterization of temperature-sensitive pantothenate kinase (coaA) mutants of Escherichia coli. J. Bacteriol. 169, 5795–5800 (1987).
Hill, J.M. & Switzer, R.C. III . The regional distribution and cellular localization of iron in the rat brain. Neuroscience 11, 595–603 (1984).
Perry, T.L.,. et al. Hallervorden-Spatz disease: cysteine accumulation and cysteine dioxygenase deficiency in the globus pallidus. Ann. Neurol. 18, 482–489 (1985).
Yoon, S.J., Koh, Y.H., Floyd, R.A. & Park, J.W. Copper, zinc superoxide dismutase enhances DNA damage and mutagenicity induced by cysteine/iron. Mutat. Res. 448, 97–104 (2000).
Park, B.E., Netsky, M.G. & Betsill, W.L. Jr . Pathogenesis of pigment and spheroid formation in Hallervorden-Spatz syndrome and related disorders. Neurology 25, 1172–1178 (1975).
Tripathi, R.C., Tripathi, B.J., Bauserman, S.C. & Park, J.K. Clinicopathologic correlation and pathogenesis of ocular and central nervous system manifestations in Hallervorden-Spatz syndrome. Acta Neuropathol. 83, 113–119 (1992).
Searle, A.J. & Willson, R.L. Stimulation of microsomal lipid peroxidation by iron and cysteine. Characterization and the role of free radicals. Biochem. J. 212, 549–554 (1983).
Yang, E.Y., Campbell, A. & Bondy, S.C. Configuration of thiols dictates their ability to promote iron-induced reactive oxygen species generation. Redox. Rep. 5, 371–375 (2000).
Heafield, M.T.,. et al. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci. Lett. 110, 216–220 (1990).
Shevell, M. Racial hygiene, active euthanasia, and Julius Hallervorden [see comments]. Neurology 42, 2214–2219 (1992).
Acknowledgements
We are grateful to the many individuals, families and clinicians (J.P. Harpey, M. Shevell, M. Piñeda, G. Kurlemann, J. Coppeto, J. Jankovic, S. Davis, H. Hattori, K. Sethi, M. Pandolfo, L. Angelini, N. Nardocci, A. Malandrini, J. Penzien, G. Mortier, M. Hoeltzenbein, J.A.Urtizberea, M.A.M. Salih, D. Buckley, C. Haenggeli, A. Bottani, B. Beinlich, J. Østergaard, S. Bundey, F. Stogbauer, K. Nørgaard Hansen, J. Guimarães, C. Yalcinkaya, A. Feigenbaum, Z. Liptai, J. Carlo, P. Blasco, A. Zimmerman, R. Cilio, E. Bertini, G. Worley, U. Thyen, J. Molineuvo, M. Melis, G. Cossu, J. Menkes, K. Hollódy, A. Barrett, S. Simpson, C. Schrander-Stumpel, H. Chaabouni, R. Gatti, H. Topaloglu, M. Nigro, F. Hisama, N.R.M. Buist, B. Ben-Ze'ev, A. Macaya, B. Korf, P. Heydemann, S. Abbs, R. Robinson, L. Shinobu, E. Dooling, P. Wheeler, P. Rosman, W. Wasiewski, P. Castelnau, P. Evrard, R. Haslam, M. Filocamo, M. Karwacki, T. Kmieæ, S. Frucht, T. Konishi, M. Regina Reyes, M. Al-Mateen, K. Weidenheim, M. Delgado, S. Johnsen, S. Golembowski, W. Ondo, S. Bohlega, R. Bustamante, O. Fernandez, M. Wiznitzer, H. Morgan, I. Butler, T. Babb, D. Sanderson, M. Williams, C. Harding, R. Steiner, S. Toor, E. Thompson, J. MacKenzie, J. Clyman and D. Fornoff) who contributed to this study. We thank T. Taylor, H. Payami, M. Litt, A. Malone, S. Bae, M. Gunthorpe, H. Consencgo, E. Stewart, S. Packman, the Oregon Health and Science University MMI Sequencing Core, and the Hallervorden–Spatz Syndrome Association. This work was supported by a grant from the National Eye Institute and the Sandler Neurogenetics Center at the University of California, San Francisco. J.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, B., Westaway, S., Levinson, B. et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 28, 345–349 (2001). https://doi.org/10.1038/ng572
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng572
This article is cited by
-
Iron imbalance in neurodegeneration
Molecular Psychiatry (2024)
-
Missing heritability of Wilson disease: a search for the uncharacterized mutations
Mammalian Genome (2023)
-
Alpha-lipoic acid supplementation corrects pathological alterations in cellular models of pantothenate kinase-associated neurodegeneration with residual PANK2 expression levels
Orphanet Journal of Rare Diseases (2023)
-
Genetic mutation spectrum of pantothenate kinase-associated neurodegeneration expanded by breakpoint sequencing in pantothenate kinase 2 gene
Orphanet Journal of Rare Diseases (2022)
-
Proton magnetic resonance spectroscopy detects cerebral metabolic derangement in a mouse model of brain coenzyme a deficiency
Journal of Translational Medicine (2022)