Abstract
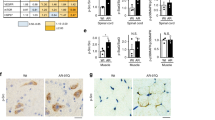
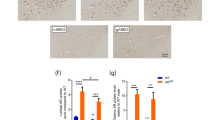
Spinal and bulbar muscular atrophy (SBMA) is an adult-onset motor neuron disease that affects males. It is caused by the expansion of a polyglutamine (polyQ) tract in androgen receptors. Female carriers are usually asymptomatic. No specific treatment has been established. Our transgenic mouse model carrying a full-length human androgen receptor with expanded polyQ has considerable gender-related motor impairment. This phenotype was abrogated by castration, which prevented nuclear translocation of mutant androgen receptors. We examined the effect of androgen-blockade drugs on our mouse model. Leuprorelin, a lutenizing hormone–releasing hormone (LHRH) agonist that reduces testosterone release from the testis, rescued motor dysfunction and nuclear accumulation of mutant androgen receptors in male transgenic mice. Moreover, leuprorelin treatment reversed the behavioral and histopathological phenotypes that were once caused by transient increases in serum testosterone. Flutamide, an androgen antagonist promoting nuclear translocation of androgen receptors, yielded no therapeutic effect. Leuprorelin thus seems to be a promising candidate for the treatment of SBMA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kennedy, W.R., Alter, M. & Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18, 671–680 (1968).
Sobue, G. et al. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112, 209–232 (1989).
Sobue, G. et al. Subclinical phenotypic expressions in heterozygous females of X-linked recessive bulbospinal neuronopathy. J. Neurol. Sci. 117, 74–78 (1993).
Mariotti, C. et al. Phenotypic manifestations associated with CAG-repeat expansion in the androgen receptor gene in male patients and heterozygous females: a clinical and molecular study of 30 families. Neuromuscul. Disord. 10, 391–397 (2000).
Schmidt, B.J., Greenberg, C.R., Allingham-Hawkins, D.J. & Spriggsm E.L. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology 59, 770–772 (2002).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Tanaka, F. et al. Founder effect in spinal and bulbar muscular atrophy (SBMA). Hum. Mol. Genet. 5, 1253–1257 (1996).
Doyu, M. et al. Severity of X-linked recessive bulbospinal neuronopathy correlates with size of the tandem CAG repeat in androgen receptor gene. Ann. Neurol. 32, 707–710 (1992).
Igarashi, S. et al. Strong correlation between the number of CAG repeats in androgen receptor genes and the clinical onset of features of spinal and bulbar muscular atrophy. Neurology 42, 2300–2302 (1992).
Zoghbi, H.Y. & Orr, H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Li, M. et al. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann. Neurol. 44, 249–254 (1998).
Li, M. et al. Nonneural nuclear inclusions of androgen receptor protein in spinal and bulbar muscular atrophy. Am. J. Pathol. 153, 695–701 (1998).
Ross, C.A. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington's disease and related disorders. Neuron 35, 819–822 (2002).
Taylor, J.P. & Fischbeck, K.H. Altered acetylation in polyglutamine disease: an opportunity for therapeutic intervention? Trends Mol. Med. 8, 195–197 (2002).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002).
Huggins, C. & Hodges, C.V. Studies on prostatic cancer. I. The effects of castration, of estrogen and of androgen injection on serum phosphates in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Labrie, F. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer 72 (suppl. 12), 3816–3827 (1993).
Yamamoto, A., Lucas, J.J. & Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101, 57–66 (2000).
Kemppainen, J.A. & Wilson, E.M. Agonist and antagonist activities of hydroxyflutamide and casodex relate to androgen receptor stabilization. Urology 48, 157–163 (1996).
Lu, S., Simon, N.G., Wang, Y. & Hu, S. Neural androgen receptor regulation: effects of androgen and antiandrogen. J. Neurobiol. 41, 505–512 (1999).
Tomura, A. et al. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J. Biol. Chem. 276, 28395–28401 (2001).
Walcott, J.L. & Merry, D.E. Ligand promotes intranuclear inclusions in a novel cell model of spinal and bulbar muscular atrophy. J. Biol. Chem. 277, 50855–50859 (2002).
Takeyama, K. et al. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35, 855–864 (2002).
Fischbeck, K.H., Lieberman, A., Bailey, C.K., Abel, A. & Merry, D.E. Androgen receptor mutation in Kennedy's disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 1075–1078 (1999).
Lieberman, A.P., Harmison, G., Strand, A.D., Olson, J.M. & Fischbeck, K.H. Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum. Mol. Genet. 11, 1967–1976 (2002).
Gottlieb, .B, Pinsky, L., Beitel, L.K. & Trifiro, M. Androgen insensitivity. Am. J. Med. Genet. 89, 210–217 (1999).
MacLean, H.E., Warne, G.L. & Zajac, J.D. Defects of androgen receptor function: from sex reversal to motor neurone disease. Mol. Cell. Endocrinol. 112, 133–141 (1995).
Kobayashi, Y. et al. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J. Biol. Chem. 275, 8772–8778 (2000).
Adachi, H et al. HSP70 chaperone over-expression ameliorates phenotypes of the SBMA transgenic mouse model by reducing nuclear-localized mutant AR protein. J. Neurosci. 23, 2203–2211 (2003).
Cummings, C..J, et al. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat. Genet. 19, 148–154 (1998).
Cummings, C.J. et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518 (2001).
McCampbell, A. et al. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc. Natl. Acad. Sci. USA 98, 15179–15184 (2001).
Steffan, J.S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Hockly, E. et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl. Acad. Sci. USA 100, 2041–2046 (2003).
Kobayashi, Y. et al. Caspase-3 cleaves the expanded androgen receptor protein of spinal and bulbar muscular atrophy in a polyglutamine repeat length-dependent manner. Biochem. Biophys. Res. Commun. 252, 145–150 (1998).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Adachi, H. et al. Transgenic mice with an expanded CAG repeat controlled by the human AR promoter show polyglutamine nuclear inclusions and neuronal dysfunction without neuronal cell death. Hum. Mol. Genet. 10, 1039–1048 (2001).
Luo, S. et al. Daily dosing with flutamide or Casodex exerts maximal antiandrogenic activity. Urology 50, 913–919 (1997).
Trottier, Y. et al. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature 378, 403–406 (1995).
Acknowledgements
We thank J. Miyazaki for providing the pCAGGS vector. This work was supported by a Center-of-Excellence grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan; by grants from the Ministry of Health, Labour and Welfare, Japan; by a grant from Naito Foundation; and by a grant from Kanae Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Katsuno, M., Adachi, H., Doyu, M. et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med 9, 768–773 (2003). https://doi.org/10.1038/nm878
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm878
This article is cited by
-
Efficacy of leuprorelin in spinal and bulbar muscular atrophy: a 3-year observational study
Neurological Sciences (2024)
-
Bicalutamide and Trehalose Ameliorate Spinal and Bulbar Muscular Atrophy Pathology in Mice
Neurotherapeutics (2023)
-
Effect of leuprorelin in bulbar function of spinal and bulbar muscular atrophy patients: observational study for 1 year
Journal of Neurology (2021)
-
Unveiling synapse pathology in spinal bulbar muscular atrophy by genome-wide transcriptome analysis of purified motor neurons derived from disease specific iPSCs
Molecular Brain (2020)
-
Exploring the Potential of Small Molecule-Based Therapeutic Approaches for Targeting Trinucleotide Repeat Disorders
Molecular Neurobiology (2020)