Abstract
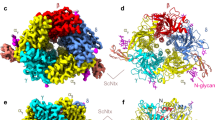
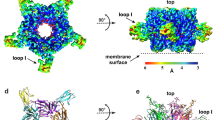
Conotoxins (Ctx) form a large family of peptide toxins from cone snail venoms that act on a broad spectrum of ion channels and receptors. The subgroup α-Ctx specifically and selectively binds to subtypes of nicotinic acetylcholine receptors (nAChRs), which are targets for treatment of several neurological disorders. Here we present the structure at a resolution of 2.4 Å of α-Ctx PnIA (A10L D14K), a potent blocker of the α7-nAChR, bound with high affinity to acetylcholine binding protein (AChBP), the prototype for the ligand-binding domains of the nAChR superfamily. α-Ctx is buried deep within the ligand-binding site and interacts with residues on both faces of adjacent subunits. The toxin itself does not change conformation, but displaces the C loop of AChBP and induces a rigid-body subunit movement. Knowledge of these contacts could facilitate the rational design of drug leads using the Ctx framework and may lead to compounds with increased receptor subtype selectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Terlau, H. & Olivera, B.M. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 84, 41–68 (2004).
Lewis, R.J. & Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2, 790–802 (2003).
McIntosh, J.M., Santos, A.D. & Olivera, B.M. Conus peptides targeted to specific nicotinic acetylcholine receptor subtypes. Annu. Rev. Biochem. 68, 59–88 (1999).
Olivera, B.M., Cruz, L.J. & Yoshikami, D. Effects of conus peptides on the behavior of mice. Curr. Opin. Neurobiol. 9, 772–777 (1999).
Miljanich, G.P. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 11, 3029–3040 (2004).
Tsetlin, V.I. & Hucho, F. Snake and snail toxins acting on nicotinic acetylcholine receptors: fundamental aspects and medical applications. FEBS Lett. 557, 9–13 (2004).
Le Novere, N., Corringer, P.J. & Changeux, J.P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456 (2002).
Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 (2002).
Smit, A.B. et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411, 261–268 (2001).
Sine, S.M., Wang, H.L. & Bren, N. Lysine scanning mutagenesis delineates structural model of the nicotinic receptor ligand binding domain. J. Biol. Chem. 13, 29210–29223 (2002).
Sine, S.M. The nicotinic receptor ligand binding domain. J. Neurobiol. 53, 431–446 (2002).
Celie, P.H. et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 (2004).
Gao, F. et al. Curariform antagonists bind in different orientations to acetylcholine-binding protein. J. Biol. Chem. 278, 23020–23026 (2003).
Hansen, S.B., Talley, T.T., Radic, Z. & Taylor, P. Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica. J. Biol. Chem. 279, 24197–24202 (2004).
Bouzat, C. et al. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430, 896–900 (2004).
Brejc, K. et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 (2001).
Cromer, B.A., Morton, C.J. & Parker, M.W. Anxiety over GABA(A) receptor structure relieved by AChBP. Trends Biochem. Sci. 27, 280–287 (2002).
Trudell, J. Unique assignment of inter-subunit association in GABA(A) α1 β3 γ2 receptors determined by molecular modeling. Biochim. Biophys. Acta 1565, 91–96 (2002).
Laube, B., Maksay, G., Schemm, R. & Betz, H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol. Sci. 23, 519–527 (2002).
Reeves, D.C. & Lummis, S.C. The molecular basis of the structure and function of the 5–HT3 receptor: a model ligand-gated ion channel (review). Mol. Membr. Biol. 19, 11–26 (2002).
O'Mara, M.L., Cromer, B., Parker, M.W. & Chung, S.H. Homology model of the GABA-A receptor examined using Brownian dynamics. Biophys. J. 88, 3286–3299 (2005).
Luo, S. et al. Single-residue alteration in α-conotoxin PnIA switches its nAChR subtype selectivity. Biochemistry 38, 14542–14548 (1999).
Hogg, R.C. et al. Single amino acid substitutions in α-conotoxin PnIA shift selectivity for subtypes of the mammalian neuronal nicotinic acetylcholine receptor. J. Biol. Chem. 274, 36559–36564 (1999).
Fainzilber, M. et al. New mollusc-specific α-conotoxins block Aplysia neuronal acetylcholine receptors. Biochemistry 33, 9523–9529 (1994).
Hogg, R.C., Hopping, G., Alewood, P.F., Adams, D.J. & Bertrand, D. α-conotoxins PnIA and [A10L]PnIA stabilize different states of the α7–L247T nicotinic acetylcholine receptor. J. Biol. Chem. 278, 26908–26914 (2003).
Bertrand, D. et al. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc. Natl. Acad. Sci. USA 89, 1261–1265 (1992).
Celie, P.H.N. et al. Crystal structure of AChBP from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J. Biol. Chem. in the press (2005); published online 16 May 2005 (10.1074/jbc.M414476200).
Hu, S.H. et al. The 1.1 Å crystal structure of the neuronal acetylcholine receptor antagonist, α-conotoxin PnIA from Conus pennaceus. Structure 4, 417–423 (1996).
Hu, S.H., Gehrmann, J., Alewood, P.F., Craik, D.J. & Martin, J.L. Crystal structure at 1.1 A resolution of α-conotoxin PnIB: comparison with α-conotoxins PnIA and GI. Biochemistry 36, 11323–11330 (1997).
Dutertre, S. & Lewis, R.J. Computational approaches to understand α-conotoxin interactions at neuronal nicotinic receptors. Eur. J. Biochem. 271, 2327–2334 (2004).
Lee, W.Y. & Sine, S.M. Invariant aspartic Acid in muscle nicotinic receptor contributes selectively to the kinetics of agonist binding. J. Gen. Physiol. 124, 555–567 (2004).
Le Novere, N., Grutter, T. & Changeux, J.P. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc. Natl. Acad. Sci. USA 99, 3210–3215 (2002).
Wolfender, J.L. et al. Identification of tyrosine sulfation in Conus pennaceus conotoxins α-PnIA and α-PnIB: further investigation of labile sulfo- and phosphopeptides by electrospray, matrix-assisted laser desorption/ionization (MALDI) and atmospheric pressure MALDI mass spectrometry. J. Mass Spectrom. 34, 447–454 (1999).
Quiram, P.A., McIntosh, J.M. & Sine, S.M. Pairwise interactions between neuronal α(7) acetylcholine receptors and α-conotoxin PnIB. J. Biol. Chem. 275, 4889–4896 (2000).
Gao, F. et al. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. J. Biol. Chem. 280, 8443–8451 (2005).
Bourne, Y., Talley, T.T., Hansen, S.B., Taylor, P. & Marchot, P. Crystal structure of a Cbtx–AChBP complex reveals essential interactions between snake α-neurotoxins and nicotinic receptors. EMBO J. 24, 1512–1522 (2005).
Schneider, T.R. A genetic algorithm for the identification of conformationally invariant regions in protein molecules. Acta Crystallogr. D 58, 195–208 (2002).
Broxton, N. et al. Leu(10) of α-conotoxin PnIB confers potency for neuronal nicotinic responses in bovine chromaffin cells. Eur. J. Pharmacol. 390, 229–236 (2000).
Unwin, N., Miyazawa, A., Li, J. & Fujiyoshi, Y. Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the α subunits. J. Mol. Biol. 319, 1165–1176 (2002).
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 424, 949–955 (2003).
Moore, M.A. & McCarthy, M.P. Snake venom toxins, unlike smaller antagonists, appear to stabilize a resting state conformation of the nicotinic acetylcholine receptor. Biochim. Biophys. Acta 1235, 336–342 (1995).
Kasheverov, I. et al. Photoactivable α-conotoxins reveal contacts with all subunits as well as antagonist-induced rearrangements in the Torpedo californica acetylcholine receptor. Eur. J. Biochem. 268, 3664–3673 (2001).
Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 346, 967–989 (2005).
Zhmak, M.N. et al. [Efficient synthesis of natural α-conotoxins and their analogs.]. Bioorg. Khim. 27, 83–88 (2001).
Perrakis, A., Morris, R. & Lamzin, V.S. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 6, 458–463 (1999).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 57, 122–133 (2001).
Ritchie, D.W. & Kemp, G.J. Protein docking using spherical polar Fourier correlations. Proteins 39, 178–194 (2000).
Morris, G.M. et al. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Acknowledgements
We thank Y. Utkin for providing snake venom toxins and fruitful discussions, beamline staff at European Synchrotron Radiation Facility Grenoble for assistance with data collection and K. Brejc and C. Ulens for critically reading the manuscript. The work was supported by a NWO-RFBR grant (A.B.S. and V.I.T.) and in part by MCB RAN grant (V.I.T.), by STW-BBC6035 (T.K.S. and A.B.S.), by NWO-CW 98016 (T.K.S.) and EU-SPINE (T.K.S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Thermodynamic parameters. (PDF 60 kb)
Supplementary Table 2
Contacts of α-conotoxin with AChBP and α7-nAChR. (PDF 92 kb)
Rights and permissions
About this article
Cite this article
Celie, P., Kasheverov, I., Mordvintsev, D. et al. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an α-conotoxin PnIA variant. Nat Struct Mol Biol 12, 582–588 (2005). https://doi.org/10.1038/nsmb951
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb951
This article is cited by
-
Origin of acetylcholine antagonism in ELIC, a bacterial pentameric ligand-gated ion channel
Communications Biology (2022)
-
Mechanism of interactions between α-conotoxin RegIIA and carbohydrates at the human α3β4 nicotinic acetylcholine receptor
Marine Life Science & Technology (2022)
-
Rigidity of loop 1 contributes to equipotency of globular and ribbon isomers of α-conotoxin AusIA
Scientific Reports (2021)
-
Structural mechanisms for α-conotoxin activity at the human α3β4 nicotinic acetylcholine receptor
Scientific Reports (2017)
-
High-Affinity α-Conotoxin PnIA Analogs Designed on the Basis of the Protein Surface Topography Method
Scientific Reports (2016)