The ferritins are a multigene family of proteins that concentrate and store iron in all prokaryotic and eukaryotic cells. 24 monomeric subunits which fold as four-helix bundles assemble to form a protein shell with 432 cubic symmetry and an external diameter of ∼130 Å. The iron is stored inside the protein shell as a mineralized core ∼80 Å in diameter. Recombinant amphibian red cell M ferritin crystallizes in ∼2 M (NH4)2SO4 at pH 4.6 in a space group that has not been reported previously. Electron microscopy, precession photography, Patterson and Fourier maps of the native protein and a UO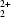 derivative, and simulations were used to determine that the unit-cell dimensions are a = b = 169.6, c = 481.2 Å, α = β = γ = 90° and the space group is P41212 or P43212. A preliminary model of the structure was obtained by molecular replacement, with amphibian red cell L ferritin as the model. In contrast to previously determined ferritin crystal structures which have intermolecular contacts at the twofold and threefold molecular axes, M ferritin crystals have a novel intermolecular interaction mediated by interdigitation of the DE loops of two molecules at the fourfold molecular axes.
derivative, and simulations were used to determine that the unit-cell dimensions are a = b = 169.6, c = 481.2 Å, α = β = γ = 90° and the space group is P41212 or P43212. A preliminary model of the structure was obtained by molecular replacement, with amphibian red cell L ferritin as the model. In contrast to previously determined ferritin crystal structures which have intermolecular contacts at the twofold and threefold molecular axes, M ferritin crystals have a novel intermolecular interaction mediated by interdigitation of the DE loops of two molecules at the fourfold molecular axes.

 journal menu
journal menu











