Abstract
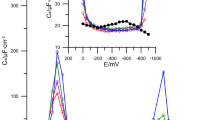
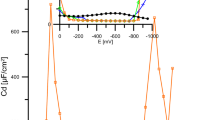
The systemn-dodecanoic acid/mercury in 0,1 N KCl solutions has been investigated within pH range 4,0–10,5 in order to characterize the adsorption of surfactant and to determine its influence on the flotation of mercury. Flotation tests have been carried out in a model mercury flotation apparatus developed by Pomianowski and Para. Some characteristic parameters of adsorption (attraction constant of Frumkin isotherm, adsorption coefficient at maximum adsorption potential, free standard adsorption enthalpy and area per molecule) have been determined by a. c. polarographic capacity-potential and capacity-time curves. Flotation recovery as well as attraction constant, adsorption coefficient and surface excess reach maximum at pH≈5,1. It is explained by the assumption of an ion-molecule associate formation.
Similar content being viewed by others
References
Akers RJ (ed) (1976) Foams Proceedings, Symposium of the Society of Chemical Industry, Colloid and Surface Chemistry Group, Brunel Univ, 8–10. 9. 1975, Academic Press, London
Schulze HJ (1981) Physikalisch-chemische Elementarvorgänge des Flotationsprozesses, p 47 ff, VEB Deutscher Verlag der Wissenschaften, Berlin
Stechemesser H, Geidel T, Weber K, Coll & Polym Sci 258:1, 109 (1980), 258:10, 1206 (1980), 259:7, 767 (1981)
Para G, Zembala M, Pomianowski A (1980) Pol J Chem 54:77
Volke K, Neubert H, Z phys Chem, in preparation
Volke K, Neubert H (1981) Z phys Chem 262:1, 39
Volke K, Neubert H (1981) Freib Forschungsh A 638:71
Para G, Volke K, Neubert H (1981) Coll & Polym Sci 259:11, 1092
Schubert H (1978) Aufb Techn 19:3, 101
Serrano A, Baldauf H, Schubert H, Bochnia D (1975) Freib Forschungsh A 544:111
Bochnia D, Serrano A, (1976) Freib Forschungsh A 565:5
Serrano A, Bochnia D, Schubert H (1977), Tenside 14:2, 67
Siebert B (1971) Freib Forschungsh A 504:4
Schubert H, Baldauf H (1978) Freib Forschungsh A 593:193
Baldauf H (1980) Freib Forschungsh A 619:5
Gaudin AM (1957) Flotation 2 ed, p 256, McGraw-Hill Book Company Inc, New York-Toronto-London
Fuerstenau MC, Miller JD (1967) Trans Soc Min Engng AIME 283:153
Du Rietz C (1958) Canad Mining J 79:8, 81
Frumkin AN (1925) Z phys Chem 116:466
Goddard ED (1974) Advan Coll Interf Sci 4:45
Nyren V, Back E (1958) Acta Chem Scand 12:6, 1305
Ralston WA (1948) Fatty acids and their derivates, vol 8, J Wiley, New York (from: Dobias B (1965) Bergakademie 17:3, 162)
Goddard ED, Ackilli JA (1963) J Coll Sci 18:585
Eagland D, Franks F (1965) Trans Faraday Soc 61:2468
Rosano HL, Breindel K, Schulman JH, Eydt AJ (1966) J Coll Interf Sci 22:1, 58
Burcik EJ, Vaughn CR (1951) J Coll Sci 6:522
Baker RH, Shafrin EG, Zisman WA (1952) J Phys Chem 56:3, 405
Merker RL, Zisman WA (1952) J Phys Chem 56:3, 399
Lucassen-Reynders EH (1973) J Coll Interf Sci 42:554
Lucassen-Reynders EH (1973) J Coll Interf Sci 42:563
Lucassen-Reynders EH (1972) J Coll Interf Sci 41:156
Lucassen-Reynders EH (1966) J Phys Chem 70:6, 1777
Lucassen-Reynders EH (1981) Anionic Surfactants, Marcel Dekker, New York
Jehring H (1974) Elektrosorptionsanalyse mit der Wechselstrompolarographie, pp 75ff, 258f, 262ff, Akademie-Verlag, Berlin
Kerker M (ed) (1976) Coll & Interf Sci, vol 5, p 262, Academic Press Inc, New York-San Francisco-London
Landolt-Börnstein (1951) Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie und Technik, 6 ed, vol 1, part 2/I, p 10, Springer-Verlag, Berlin-Heidelberg-New York
Markley KS (ed) (1960), Fatty acids: Their chemistry, properties, production and uses, 2 ed, vol 1, pp 298 and 304, Interscience Publishers, New York
Smith T (1972) Advan Coll Interf Sci 3:161
Mukerjee P (1967) Advan Coll Interf Sci 1:3, 241
Orzechowska M, Matysik J (1979) J Electroanal Chem 103:251
Lorenz W (1958) Z Elektrochem 62:2, 192
Baikerikar KG, Sathyanarayana S (1970) J Coll Electroanal Chem 24:333
Goddard ED, Kung HC (1971), J Coll Interf Sci 37:585
Goddard ED, Kao O, Kung HC (1967) J Coll Interf Sci 24:297
Van Voorst Vader F (1961) Trans Faraday Soc 57:2263
Majofis AD (1979) Kand diss LTU 1977, taken from: Powierchnostno-aktiwnye wieszczestwa, Sprawocznik, Izd Chimia Leningrad
Arcuri C (1966) Verh Kon Vlaam Acad Wetensch Lett Schone Kunsten Belg, A 162, 149 — from [31]
Author information
Authors and Affiliations
Additional information
Publ.-No. 810 from Research Institute of Mineral Processing, Academy of Sciences of the German Democratic Republic, Freiberg
Rights and permissions
About this article
Cite this article
Volke, K., Para, G., Pawlikowska-Czubak, J. et al. Flotation and adsorption investigations of the systemn-dodecanoic acid/mercury at different pH values. Colloid & Polymer Sci 262, 245–251 (1984). https://doi.org/10.1007/BF01458968
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01458968