Summary
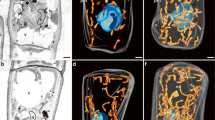
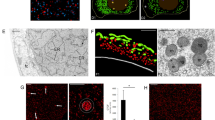
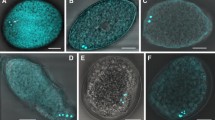
The preferential development of giant mitochondria and their nuclei (nucleoids) in the egg cells ofPelargonium zonale Ait. during megasporogenesis and megagametogenesis was examined by fluorescence microscopy, after Technovit embedding and 4′,6-diamidino-2-phenylindole (DAPI) staining, fluorimetry for DNA content, using a video-intensified microscope photon-counting system (VIMPICS), and by three-dimensional reconstruction of mitochondrial nuclei (mt-nuclei). Reproductive cells during the megaspore mother cell, meiosis, tetrad, and functioning megaspore stages contained many small mitochondria with characteristic, uniformly DAPI-stained mt-nuclei about 0.3 μm in diameter, containing a small amount of DNA (0.3 Mbp). During formation of the 2-, 4-, and 8-nucleate embryo sac, mt-nuclei did not markedly change in shape or DNA content. When the embryo sac formed and differentiation of each cell began, mitochondria and their nuclei in the egg cell took on a small ring or string-like shape. Accompanying the maturation of the embryo sac, they underwent progressive enlargement and gradually altered to long thick strings, or stacks of concentric or half concentric rings. By flower opening, they have developed to an extremely large size. One of these stacks of mt-nuclei was reconstructed in three dimensions; each ring in the stack was cup- or plate-shaped; 5 to 10 rings made up the stack, though each remained discontinuous from the others. From serial sections, we counted 44 mitochondria in one egg cell. Fluorometry using VIMPICS revealed that DNA amount within the stacked mitochondrion increased to 40 times that of the megaspore mother cell stage; a single stack of mitochondria contained 340–1700 Mbp DNA; which means that one egg cell contains at least 15000 Mbp mt-DNA, a value greater than the cell-nuclear DNA content.
Similar content being viewed by others
References
Bromberg R (1974) Mitochondrial fragmentation during germination inBlastodadiella emersonii. Dev Biol 36: 187–194
Burr FA, West JA (1970) Light and electron microscope observations on the vegetative and reproductive structures ofBryopsis hypnoides. Phycologia 9: 17–37
Chida Y, Ueda K (1986) Mitochondrial number and form change during autospore formation inChlorococcum infusionum (Schrank) Meneghini (Chlorococcales, Chlorophyta). Phycologia 25: 503–509
Diboll AG, Larson DA (1966) An electron microscopic study of the mature megagametophyte inZea mays. Am J Bot 53: 391–402
— (1968) Fine structural development of the megagametophyte inZea mays following fertilization. Am J Bot 53: 391–402
Duckett JG (1973) An ultrastructural study on the differentiation of the spermatozoid ofEquisetum. J Cell Sci 12: 95–129
— (1975) Spermatogenesis in pteridophytes. In: Duckett JG, Racey PA (eds) The biology of the male gamete. Academic Press, London, pp 97–128
Faure JE, Mogensen HL, Kranze E, Digonnet C, Dumas C (1992) Ultrastructural characterization and three-dimensional reconstruction of isolated maize (Zea mays L.) egg cell protoplasts. Protoplasma 171: 97–103
Freifelder D (1970) Molecular weights of coliphages and coliphage DNA IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of MJ. Mol Biol 54: 567–577
Fujie M, Kuroiwa H, Kawano H, Kuroiwa T (1993) Studies on the behavior of organelles and their nucleoids in the root apical meristem ofArabidopsis thaliana (L.) Col. Planta 189: 443–452
Jensen WA (1965) The ultrastructure and composition of the egg and central cell of cotton. Am J Bot 52: 781–797
Jonson KA, Rosenbaum JL (1990) The basal bodies ofChlamydomonas reinhardtii do not contain immunologically detectable DNA. Cell 62: 615–619
Kawano S, Takano H, Mori K, Kuroiwa T (1991) A mitochondrial plasmid that promotes mitochondrial fusion inPhysarum polycephalum. Protoplasma 160: 167–169
Kuroiwa H (1991) The application of the Technovit embedding method for the research of plant embryology. Plant Morphol 3: 43–47
—, Kuroiwa T (1992) Giant mitochondria in the mature egg cell ofPelargonium zonale. Protoplasma 168: 184–188
Kuroiwa T, Suzuki T, Ogawa K, Kawano S (1981) The chloroplast nucleus: distribution, number, size, and shape, and a model for the multiplication of the chloroplast genome during chloroplast development. Plant Cell Physiol 22: 381–396
—, Nakamura S, Kawano S, Hizume M, Toh-e A, Miyakawa I, Sando N (1986) Characterization of a synaptonemal complex-less nucleolar-organizing region bivalent in yeastSaccharomyces cerevisiae using a video intensified microscope system. Exp Cell Res 165: 199–206
—, Kuroiwa H, Mita T, Fujie M (1990) Fluorescence microscopic study of the formation of giant mitochondrial nuclei in the young ovules ofPelargonium zonale. Protoplasma 158: 191–194
—, Fujie M, Kuroiwa H (1992) Studies on the behavior of mitochondrial DNA. Synthesis of mitochondrial DNA occurs actively in a specific region just above the quiescent center in the root meristem ofPelargonium zonale. J Cell Sci 101: 483–493
McConchie CA, Hough T, Knox RB (1987) Ultrastructural analysis of the sperm cells of mature pollen of maize,Zea mays. Protoplasma 139: 9–19
Mogensen HL, Suthar HK (1979) Ultrastructure of the egg apparatus ofNicotiana tabacum (Solanaceae) before and after fertilization. Bot Gaz 140: 168–179
Myles DG, Bell PR (1975) An ultrastructural study of the spermatozoid of the fernMarsilea vestita. J Cell Sci 17: 633–645
Nishibayashi S, Kawano S, Kuroiwa T (1987) Light and electron microscopic observations of mitochondrial fusion in plasmodia induced sporulation inPhysarum polycephalum. Cytologia 52: 599–614
Osafune T, Mihara S, Hase E, Ohkuro I (1975) Formation and division of giant mitochondria during the cell cycle ofEuglena gracilis Z in synchronous culture I. Some characteristics of changes in the morphology of mitochondria and oxygen-uptake activity of cells. Plant Cell Physiol 16: 313–326
—, Sumida S, Ehara T, Hase E (1989) Three-dimensional distribution of ribulose-1,5-biphosphate carboxylase/oxygenase in chloroplasts of actively photosynthesizing cell ofEuglena gracilis. J Electron Microsc 38: 399–402
Remacle C, Bovie C, Michel-Wolwertz MR, Loppes R, Matagne RF (1990) Mitochondrial genome transmission inChlamydomonas diploids obtained by sexual crosses and artificial fusions: role of the mating type and of a 1 kb intron. Mol Gen Genet 223: 180–184
Rusche ML, Mogensen HL (1988) The male germ unit ofZea mays: quantitative ultrastructure and three-dimensional analysis. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York Tokyo, pp 221–226
Sando N, Miyakawa S, Nishibayashi S, Kuroiwa T (1981) Arrangement of mitochondrial nucleoids in life cycle ofSaccharomyces cerevisiae. J Gen Appl Microbiol 27: 511–516
Schulz P, Jensen WA (1968) Capsella embryogenesis: the egg, zygote, and early embryo. Am J Bot 55: 807–819
— — (1973) Capsella embryogenesis: the central cell. J Cell Sci 12: 741–763
Suzuki T, Kawano S, Sakai A, Fujie M, Kuroiwa H, Kuroiwa T (1992) Preferential mitochondrial and plastid DNA synthesis before multiple cell divisions inNicotiana tabacum. J Cell Sci 103: 831–837
- Sakai A, Kawano S, Kuroiwa T (1996) Preferential organelle DNA synthesis before cell nuclear replication is essential for subsequent cell propagation. Cytologia (in press)
Tanaka K, Kanbe T, Kuroiwa T (1985) Three-dimensional behaviour of mitochondria during cell division and germ tube formation in the dimorphic yeastCandida albicans. J Cell Sci 73: 207–220
Tourte Y (1975) Etude infrastructurale de l'oogenese chez une pteridophyte. II. Evolution des mitochondries et des plastes. J Microsc Biol Cell 23: 301–316
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuroiwa, H., Ohta, T. & Kuroiwa, T. Studies on the development and three-dimensional reconstruction of giant mitochondria and their nuclei in egg cells ofPelargonium zonale Ait.. Protoplasma 192, 235–244 (1996). https://doi.org/10.1007/BF01273895
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01273895