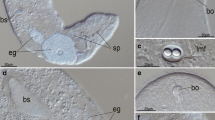
Summary
A combination of microscopical, immunocytochemical, and autoradiographic techniques were employed to study stem cells and their fates during asexual reproduction and regeneration in two microturbellarians,Microstomum lineare (Macrostomida) andStenostomum leucops (Catenulida). Special attention was paid to the development of the immunoreactivity (IR) to FMRF/RF-amide and 5-HT in differentiating nerve cells.
Asexual reproduction inM. lineare andS. leucops occurs by paratomy, i.e., fragmentation after completed differentiation of the new organs. Regeneration, on the other hand, involves a combination of morphallactic and epimorphic processes without the formation of a regeneration blastema. The only cells incorporating tritiated thymidine ([3H]T) were the mesenchymal and gastrodermal neoblasts, which proliferate continuously replenishing the population of stem cells available for growth, asexual reproduction and regeneration. These proliferative cells occurred in two ultrastructurally different forms, differing from each other only by the presence or absence of ciliar basal bodies in the cytoplasm. Few differentiated cells were labeled in the head piece after completed regeneration. A greater amount of labeled differentiated cells were, however, observed postpharyngeally in the first zooid as well as in zooids having developed during the same time (i.e., 20–45 h after the treatment with [3H]T). Furthermore, many labeled cells were still undifferentiated at that time or just in the beginning of the differentiation process. It can therefore be concluded that neoblasts function both as reserve cells and as functional stem cells for all differentiated cell types in these worms. IR to FMRF/RF-amide neuropeptides was not observed in nerve cells differentiating from neoblasts until the occurrence of dense-core vesicles in their cytoplasm. Due to methodological difficulties only weak or no IR to 5-HT could be traced in the nervous system of the asexual and regenerating worms.
Similar content being viewed by others
Abbreviations
- ICC:
-
Immunocytochemical
- IR:
-
immunoreactivity
- [3H]T:
-
tritiated thymidine
References
Baguñà J, Saló E, Auladell C (1989 a) Regeneration and pattern formation in planarians. III. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. Development 107: 77–86
— —, Romero R (1989 b) Effects of activators and antagonists of the neuropeptides substance P and substance K on cell proliferation in planarians. Int J Dev Biol 33: 261–264
Best JB, Rosenvold R, Souders J, Wade C (1965) Studies on the incorporation of isotopically labeled nucleotides and amino acids in planaria. J Exp Zool 159: 397–403
Chan-Palay V, Palay SL (1984) Coexistence of neuroactive substances in neurons. Wiley, New York
Coward SJ, Bennett CE, Hazlehurst BL (1974) Lysosomes and lysosomal enzyme activity in the regenerating planarian; evidence in support of dedifferentiation. J Exp Zool 189: 133–145
Drobysheva IM (1986) Physiological regeneration of the digestive parenchyme inConvoluta convoluta andOxyposthia praedator (Turbellaria, Acoela). Hydrobiologia 132: 189–193
— (1988) An autoradiographic study of the replacement of epidermis in polyclad turbellarians. Fortschr Zool 38: 97–101
Franquinet R, Moraczewski J, LeMoigne A (1985) Phosphorylation of endogenous proteins, including histones, during initiation of planarian regeneration. Comp Biochem Physiol 80 B: 661–669
Friedel T, Webb RA (1979) Stimulation of mitoses inDugesia tigrina by a neurosecretory fraction. Can J Zool 57: 1818–1819
Fujita T, Kanno T, Kobayashi S (1988) The paraneuron. Springer, Tokyo Berlin Heidelberg New York
Gremigni V (1988) Planarian regeneration: an overview of some cellular mechanisms. Zool Sci 5: 1153–1163
—, Miceli C, Picano E (1980) On the role of germ cells in planarian regeneration. II Cytophotometric analysis of the nuclear Feulgen-DNA content in cells of regenerated somatic tissues. J Embryol Exp Morphol 55: 65–76
Hyman LH (1951) The invertebrates, vol II, platyhelminthes and rhynchocoela. McGraw-Hill, New York
Jurand A, Goel SC (1976) The use of methyl green-pyronin staining after glutaraldehyde fixation and paraffin or araldite embedding. Tissue Cell 8: 389–394
Kurabuchi S, Kishida Y (1979) The role of the nervous system in the planarian regeneration III. The influence of the head and nerve cords on the blastema regeneration. Annot Zool Japon 52: 179–187
Lange CS (1983) Stem cells in planarians. In: Potten CS (ed) Stem cells. Churchill Livingstone, Edinburgh, pp 28–66
Lender T (1974) The role of neurosecretion in freshwater planarians. In: Riser NW, Morse HP (eds) Biology of theTurbellaria. McGraw-Hill, New York, pp 460–475
Martelly I, Franquinet R, LeMoigne A (1981) Relationships between variations of cAMP, neuromediators and the stimulation of nucleic acid synthesis during planarian (Polycelis tenuis) regeneration. Hydrobiologia 84: 195–201
Moraczewski J (1977) Asexual reproduction and regeneration ofCatenula (Turbellaria, Archoophora). Zoomorphologie 88: 65–80
—, Martelly I, Franquinet R, Castagna M (1987) Protein kinase C activity during planarian regeneration. Comp Biochem Physiol 87 B: 703–707
Morita M, Best JB (1984) Electron microscopic studies of planarian regeneration. IV. Cell division of neoblasts inDugesia doroto cephala. J Exp Zool 229: 425–436
Nentwig MR (1978) Comparative morphological studies of head development after decapitation and after fission in the planarian Dugesia dorotocephala. Trans Am Microsc Soc 97: 297–310
Palmberg I (1986) Cell migration and differentiation during wound healing and regeneration inMicrostomum lineare (Turbellaria). Hydrobiologia 132: 181–188
—, Reuter M (1983) Asexual reproduction inMicrostomum lineare (Turbellaria). I. An autoradiographic and ultrastructural study. Int J Invertebr Reprod 6: 197–206
— — (1990) Neuronal subsets in regeneratingMicrostomum lineare. Immunocytochemistry of 5-HT and RF-amide. In: Gustafsson MKS, Reuter M (eds) The early brain. Proceedings of the symposium on invertebrate neurobiology. Åbo Akademi University, 19–20 September 1989. Åbo Akademi Press, Åbo, pp 147–160
Reuter M (1990) From innovation to integration. Trends of the integrative systems in microturbellarians. In: Gustafsson MKS, Reuter M (eds) The early brain. Proceedings of the symposium on invertebrate neurobiology. Åbo Akademi University, 19–20 September 1989, Åbo Akademi Press, Åbo, pp 161–178
—, Gustafsson M (1989) “Neuroendrocrine cells” in flatworms — progenitors to metazoan neurons. Arch Histol Cytol 52: 253–263
—, Palmberg I (1983) Asexual reproduction inMicrostomum lineare (Turbellaria). II. The nervous system in the division zone. Int J Invertebr Reprod 6: 207–217
— — (1987) An ultrastructural and immunocytochemical study of gastrodermal cell types inMicrostomum lineare (Turbellaria, Macrostomida). Acta Zool (Stockh) 68: 153–163
— — (1989) Development and differentiation of neuronal subsets in asexually reproducingMicrostomum lineare. Immunocytochemistry of 5-HT, RF-amide and SCPB. Histochemistry 91: 123–131
—, Karhi T, Schot LPC (1984) Immunocytochemical demonstration of peptidergic neurons in the central and peripheral nervous systems of the flatwormMicrostomum lineare with antiserum to FMRF-amide. Cell Tissue Res 238: 431–436
—, Lehtonen M, Wikgren M (1988) Immunocytochemical evidence of neuroactive substances in flatworms of different taxa — a comparison. Acta Zool (Stockh) 69: 29–37
—, Wikgren M, Lehtonen M (1986) Immunocytochemical demonstration of 5-HT-like and FMFR-amide-like substances in whole mounts ofMicrostomum lineare (Turbellaria). Cell Tissue Res 246: 7–12
— —, Palmberg I (1980) Thenervous system ofMicrostomum lineare (Turbellaria, Macrostomida) I. A fluorescence and electron microscopic study. Cell Tissue Res 211: 31–40
Saló E, Baguñà J (1984) Regeneration and pattern formation in planarians. I. The pattern of mitosis in anterior and posterior regeneration inDugesia (G)tigrina, and a new proposal for blastema formation. J Embryol Exp Morphol 83: 63–80
— — (1985) Cell movement in intact and regenerating planarians. Quantitation using chromosomal, nuclear and cytoplasmic markers. J Embryol Exp Morphol 89: 57–70
— — (1989 a) Regeneration and pattern formation in planarians. II. Local origin and role of cell movements in blastema formation. Development 107: 69–76
Saló E, Baguñà J (1989 b) Changes in ornithine decarboxylase activity and polyamine content and effects of polyamine inhibitors in the regenerating planarianDugesia (G)tigrina. J Exp Zool 250: 150–161
Sternberger LA (1979) Immunocytochemistry, 2 edn. Prentice Hall, Englewood Cliffs, NJ
Stubblefield E (1981) Cell cycle. In: Schwartz LM, Azar MM (eds) Advanced cell biology. Van Nostrand Reinhold, New York, pp 1005–1025
Varndell IM, Polak JM (1984) Double immunostaining procedures: Techniques and applications. In: Polak JM, Varndell IM (eds) Immunolabelling for electron microscopy. Elsevier, Amsterdam, pp 155–177
Webb RA (1988) Endocrinology of acoelomates. In: Endocrinology of selected invertebrate types. Alan R Liss, New York, pp 31–62
Wikgren M, Reuter M (1985) Neuropeptides in a microturbellarian — whole-mount immunocytochemistry. Peptides 6 [Suppl 3]: 471–475
— —, Gustafsson M (1986) Neuropeptides in free-living and parasitic flatworms (Platyhelminthes). An immunocytochemical study. Hydrobiologia 132: 93–99
Williams MA (1980) Autoradiography and immunocytochemistry. In: Glauert MA (ed) Practical methods in electron microscopy. North-Holland, Amsterdam, pp 1–76
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palmberg, I. Stem cells in microturbellarians. Protoplasma 158, 109–120 (1990). https://doi.org/10.1007/BF01323123
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323123