Summary
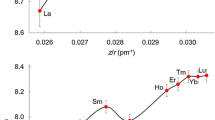
The thermodynamic stepwise formation constants (logT K n) of nine tervalent lanthanons (La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+, Dy3+, Er3+ and Lu3+) with three fluorinated β-ketoesters (methyltrifluroacetoacetate, ethyltrifluoroacetoacetate, and ethylpentafluoropropionylacetate) have been evaluated potentiometrically in a 50% dioxane-water mixture at 25 and 35 ± 0.01 °C. The values of logT K n do not follow a linearity when plotted againstZ/r and invariably obey the sequence: La3+ < Nd3+ < Pr3+ < Sm3+ < Gd3+ < Eu3+ < Dy3+ ≤ Er3+ ≤ Lu3+ in all instances. The standard thermodynamic parameters (ΔG 01 , ΔH 01 , ΔS 01 ) associated with logT K n have also been calculated. The validity of the chosen equilibrium model was examined by an error analysis usingS min values (sum of the squared residuals), scatter plots, and slopes and intercepts of Abrahams-Kave type normal probability plots.
Zusammenfassung
Es wurden die thermodynamischen stufenweisen Komplexbildungskonstanten (logT K n) von 9 dreiwertigen Lanthanoiden (La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+, Dy3+, Er3+ und Lu3+) mit drei fluorierten β-Ketoestern (Methyltrifluoracetoacetat, Ethyltrifluoracetoacetat und Ethylpentafluopropionylacetat) in 50% Dioxan-Wasser bei 25 und 35±0.01 °C potentiometrisch bestimmt. Die Werte für logT K n ergeben keine lineare Abhängigkeit gegenüberZ/r, sie gehorchen stets der Reihenfolge: La3+ < Nd3+ < Pr3+ < Sm3+ < Gd3+ < Eu3+ < Dy3+ ≤ Er3+ ≤ Lu3+. Die thermodynamischen Standardparameter ΔG 01 , ΔH 01 und ΔS 01 wurden ebenfalls berechnet. Die Gültigkeit des gewählten Gleichgewichtsmodells wurde unter Verwendung der Summe der Quadratreste (S min), von Streukurven und Steigung/Ordinatenabschnitt der Normalwahrscheinlichkeitsdarstellung nach Abrahams-Kave untersucht.
Similar content being viewed by others
References
Eur. Pat. Appl. EP. 306,929, 15 Mar. (1989) [cf. (1989) Chem. Abstr.111: 133996m]
Jpn., Kokai, Tokkyo, JP., 01,313,406, 18 Dec. (1989) [cf. (1990) Chem. Abstr.113: 36396z]
Eur. Pat. Appl. EP. 389,499, 22 Nov. (1990) [cf. (1991) Chem. Abstr.114: 164269t]
Ahrland S. (1968) Structure and Bonding, Vol. 5. New York: Springer
Gran G. (1952) Analyst77: 61
Weissberger A., Proskauer E. S. (1955) Organic Solvents, Vol. 7. New York: Interscience, p. 139
Shukla J. P., Sharma R. S. (1990) J. Prakt. Chem.332: 619
Van Uitert L. G., Haas C. G. (1953) J. Am. Chem. Soc.75: 451
Shukla J. P., Tandon S. G. (1972) J. Electroanal. Chem.35: 423
Harned H. S., Owen B. B. (1958) The Physical Chemistry of Electrolytic Solutions. New York: Reinhold, p. 713
Izatt R. M., Haas C. G., Block B. P., Fernelius W. C. (1954) J. Phys. Chem.58: 1133
Harned H. S., Owen B. B. (1958) The Physical Chemistry of Electrolytic Solutions. New York: Reinhold, p. 163
Schaefer W. P. (1962) Inorg. Chem.4: 642
Ting-Po I., Nancollas G. H. (1972) Anal. Chem.44: 1940
Avdeef A., Sofen S. R., Bregante T. L., Raymond K. N. (1978) J. Am. Chem. Soc.100: 5362
Abrahams S. C., Keve E. T. (1971) Acta Crystallogr.A27: 157
Dutt N. K., Rahut S. (1971) J. Inorg. Nucl. Chem.33: 1725
Dutt N. K., Rahut S. (1970) J. Inorg. Nucl. Chem.32: 2905
Choppin G. R., Chebaugh E. (1978) J. Inorg. Chem.17: 2301
Grenthe I., Fernelius W. C. (1960) J. Am. Chem. Soc.82: 6258
Sinha S. P. (1976) Structure and Bonding, Vol. 30. New York: Springer
Sinha S. P. (1976) Structure and Bonding, Vol. 25. New York: Springer
Dzhurinskii B. F. (1980) Russ. J. Inorg. Chem. (Eng.)25: 41
Williams R. J. P. (1982) Structure and Bonding, Vol. 50. New York: Springer, p. 81
Mathur J. N., Pai S. A., Khopkar P. K., Subramanian M. S. (1977) J. Inorg. Nucl. Chem.39: 653
Kertes A. S., Kassierer E. F. (1972) Inorg. Chem.11: 2108
Martin D. F., Moeller T., Randall W. J. (1982) J. Ind. Chem. Soc.59: 1232
Perrin D. D., Syce I. G. (1967) Talanta14: 833
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shukla, J.P., Sharma, R.S. Study on complex formation equilibria of tervalent lanthanons with fluorinated β-ketoesters in aqueous dioxane medium. Monatsh Chem 125, 247–258 (1994). https://doi.org/10.1007/BF00811310
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00811310