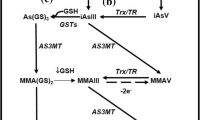
Abstract
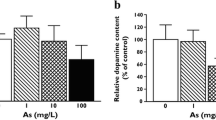
Benzene, a common groundwater contaminant, possesses neurotoxic and behavioral effects. Male, adult CD-1 mice were continuously fed drinking waterad libitum containing 0, 31, 166 and 790 mg/L benzene for four weeks. Endogenous levels of the catecholamines norepinephrine (NE) and dopamine (DA), the catecholamine metabolites 3-methoxy-4-hydroxymandelic acid (VMA), 3,4-di-hydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and the indoleamine serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), were measured by high-performance liquid chromatography (HPLC) in six discrete brain regions. In the hypothalamus, the brain region richest in NE, concentrations of NE increased by 40, 58 and 61% when mice received doses at 31, 166 and 790 mg/L, respectively. Significant increases of NE were also observed in the medulla oblongata and cerebellum. Dopamine concentrations increased significantly in the hypothalamus and corpus striatum. Increases of catecholamine metabolites were seen in a number of brain regions: midbrain (DOPAC), corpus striatum (VMA, DOPAC, HVA), cerebral cortex (VMA) and cerebellum (VMA). Benzene ingestion significantly increased 5-HT concentrations in the hypothalamus, corpus striatum, midbrain, cerebral cortex and medulla oblongata. Concomitant with increases of 5-HT, 5-HIAA increased in corpus striatum, midbrain, cerebral cortex and medulla oblongata. The findings indicate that oral ingestion of benzene by CD-1 mice induced both synthesis and catabolism of the monoamine neurotransmitters investigated.
Similar content being viewed by others
References
Baslo A, Aksoy M (1982) Neurological abnormalities in chronic benzene poisoning. A study of six patients with aplastic anemia and two with preleukemia. Environ Res 27:457–465
Bolcsak LE, Nerland DE (1983) Inhibition of erythropoiesis by benzene metabolites. Toxicol Appl Pharmacol 69:363–368
Brief RS, Lynch J, Bernath T, Scala RA (1980) Benzene in the workplace. Am Ind Hyg Assoc J 41:616–623
Claman HN (1972) Corticosteroids and lymphoid cells. New England J Med 287:388–397
Commissiong JW (1985) Monoamine metabolites: Their relationship and lack of relationship to monoaminergic neuronal activity. Biochem Pharmacol 34:1127–1131
Cooper JR, Bloom FE, Roth RH (1986) The Biochemical Basis of Neuropharmacology. Oxford University Press, New York
Coulombe RA Jr, Sharma RP (1986) Neurobiochemical alterations induced by the artificial sweetner aspartame (Nutra-Sweet). Toxicol Appl Pharmacol 83:79–85
Dempster AM, Evans HL, Snyder CA (1984) The temporal relationship between behavioral and hematological effects of inhaled benzene. Toxicol Appl Pharmacol 76:195–203
Dowdy S, Wearden S (1983) Statistics for Research. John Wiley & Sons, New York
Faiman MD, Myers NB, Schowen RL (1973) Post-mortem degradation kinetics of norepinephrine. Biochem Pharmacol 22:2171–2181
Fishbein L (1984) An overview of environmental and toxicological aspects of aromatic hydrocarbons. I. Benzene. Sci Total Environ 40:189–218
Glowinski J, Iverson LL (1966) Regional studies of catecholamines in the rat brain. J Neurochem 13:655–669
Grasso P, Sharratt M, Davies DM, Irvine D (1984) Neurological and psychological disorders and occupational exposure to organic solvents. Fd Chem Toxicol 22:819–852
Greenlee WF, Sun JD, Bus JS (1981) A proposed mechanism of benzene toxicity: formation of reactive intermediates from polyphenol metabolites. Toxicol Appl Pharmacol 59:187–195
Haley TJ (1977) Evaluation of the health effects of benzene inhalation. Clin Toxicol 11:531–548
Hsieh GC, Sharma RP, Parker RDR (1988) Subclinical effects of groundwater contaminants. I. Alteration of humoral and cellular immunity by benzene in CD-1 mice. Arch Environ Contamin Toxicol 17:151–158
Irons RD (1985) Quinones as toxic metabolites of benzene. J Toxicol Environ Hlth 16:673–678
Juorio AV, Yu PH (1985) Effects of benzene and pyridine on the concentration of mouse striatal tryptamine and 5-hydroxytryptamine. Biochem Pharmacol 34:3774–3776
— (1985a) Effects of benzene and other organic solvents on the decarboxylation of some brain aromatic-L-amino acids. Biochem Pharmacol 34:3381–1387
Leibowitz SF (1986) Brain monoamines and peptides: Role in the control of eating behavior. Federation Proc 45:1396–1403
Makara GB, Palkovits M, Szentagothai (1980) The endocrine hypothalamus and the hormonal response to stress. In: Selye H (ed) Selye's Guide to Stress Research. Van Nostrand Reinhold, New York, pp 280–337
Mayer GS, Shoup RE (1983) Simultaneous multiple electrode liquid chromatographic-electrochemical assay for catecholamines, indoleamines and metabolites in brain tissue. J Chromatogr 255:533–544
Paradowski M, Heimburger M, Cohen Y (1984) The effects of a single dose and prolonged benzene administration on 5-hydroxytryptamine and 5-hydroxyindoleacetic acid content in rat brain. Xenobiotica 14:781–784
Paradowski M, Prioux-Guyonneau M, Heimburger M, Andrzejewski SW, Cohen Y (1985) Effects of single and prolonged benzene administration on noradrenaline and dopamine content of the rat brain and internal organs. Biog Amines 2:191–196
Poirier LJ, Bedard PJ (1984) Behaviour correlates of neurotransmitter activity. Can J Neurol Sci 11:100–104
Rogawski MA, Baker JL (1985) Neurotransmitter Actions in the Vertebrate Nervous Systems. Plenum Press, New York
Savolainen H (1977) Some aspects of the mechanisms by which industrial solvents produce neurotoxic effects. Chem Biol Interact 18:1–10
Sawahata T, Rickert DE, Greenlee WF (1986) Metabolism of benzene and its metabolites in bone marrow. In: Irons RD (ed) Toxicology of the Blood and Bone Marrow. Raven Press, New York, pp 141–148
Sharma RP, Coulombe RA, Srisuchart B (1986) Effects of dietary vanadium exposure on levels of regional brain neurotransmitters and their metabolites. Biochem Pharmacol 35:461–465
US Environmental Protection Agency (1980) Ambient Water Quality Criteria for Benzene. (EPA 440/5-80-018). Washington DC
-(1982) Test Methods for Evaluating Solid Waste. Physical/Chemical Methods. 2nd Edition. Office of Solid Waste and Emergency Response. SW-846. Washington DC
Willis GL, Smith GC (1985) Amine accumulation in behavioural pathology. Brain Res Rev 9:109–132
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hsieh, G.C., Parker, R.D.R. & Sharma, R.P. Subclinical effects of groundwater contaminants. II. Alteration of regional brain monoamine neurotransmitters by benzene in CD-1 mice. Arch. Environ. Contam. Toxicol. 17, 799–805 (1988). https://doi.org/10.1007/BF01061984
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01061984