Summary
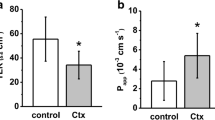
The physiological relevance of an apparent ionophore activity of cholera toxin towards Ca2+ has been examined in several different systems designed to measure affinity, specificity, rates of ion transfer, and effects on intracellular ion concentrations. Half-maximal transfer rates across porcine jejunal brush-border vesicles were obtained at a concentration of 0.20 μM Ca2+. When examined in the presence of competing ions the transfer process was blocked by very low concentrations of La3+ or Cd2+. Sr2+, Ba2+ and Mg2+ were relatively inefficient competitors for Ca2+ transport mediated by cholera toxin. The relative affinities observed would be compatible with a selectivity for Ca2+ transfer at physiological ion concentrations, as well as an inhibition of this ionophore activity by recognized antagonists of cholera toxin such as lanthanum ions. Entry rates of Ca2+ into brush-border vesicles exposed to cholera toxin were large enough to accelerate the collapse of a Ca2+ gradient generated by endogenous Ca, Mg-ATPase activity. The treatment of isolated jejunal enterocytes with cholera toxin caused a significant elevation in cytosolic Ca2+ concentrations as measured by Quin-2 fluorescence. This effect was specifically prevented by prior exposure of the cholera toxin to excess ganglioside GM1. We conclude that cholera toxin has many of the properties required for promoting transmembranes Ca2+ movement in membrane vesicles and appears to be an effective Ca2+ ionophore in isolated mammalian cells.
Similar content being viewed by others
References
Bartfai, T. 1979. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes.Int Advances in Cyclic Nucleotide Research, Vol. 10. G. Brooker, P. Greengard, and G.A. Robinson, editors. Raven, New York
Donowitz, M., Charney, A.N., Hynes, R. 1979. Propranolol inhibition of cholera enterotoxin-induced intestinal secretion in the rat.Gastroenterology 76:482–491
Farack, V.M., Kautz, V., Loechke, R. 1981. Loperamide reduces the intestinal secretion but not the cAMP accumulation induced by cholera toxin.Naunym-Schmiedeberg's Arch. Pharmacol. 137:178–179
Field, M. 1971. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3′, 5′-AMP.Am. J. Physiol. 221:992–997
Fishman, P.H., Atikkan, E.E. 1980. Mechanism of action of cholera toxin: Effect of receptor density and multivalent binding on activation of adenylate cyclase.J. Membrane Biol. 54:51–60.
Forsyth, G.W., Hamilton, D.L., Goertz, K.E., Johnson, M.R. 1978. Cholera toxin effects on fluid secretion, adenylate cyclase and cyclic AMP in porcine small intestine.Infection & Immunity 21:373–380
Forsyth, G.W., Hamilton, D.L., Scoot, A., Goertz, K.E. 1979. Failure to reverse cholera toxin-induced intestinal secretion by agents which decrease mucosal cyclic AMP.Can. J. Physiol. Pharmacol. 57:1004–1010
Forsyth, G.W., Kapitany, R.A., Scoot, A. 1981. Nicotinic acid inhibits enterotoxin-induced jejunal secretion in the pig.Can. J. Comp. Med. 45:167–172
Hamilton, D.L., Johnson, M.R., Forsyth, G.W., Roe, W.E., Nielsen, N.O. 1978. The effect of cholera toxin and heat labile and heat stable enterotoxin ofEscherichia coli on cyclic AMP concentrations in small intestinal mucosa of pig and rabbit.Can J. Comp. Med. 42:327–331
Holmgren, J., Lonnroth, I. 1976. Cholera toxin and the adenylate cyclase activating signal.J. Infect. Dis. 133:S64-S74
Knoop, F.C., Thomas, D.D. 1984. Effect of cholera enterotoxin on calcium uptake and cyclic AMP accumulation in rat basophilic leukemia cells.Int. J. Biochem. 16:275–280
Leitch, G.J., Amer, M.S. 1975. Lanthanum inhibition ofVibrio cholerae andEscherichia coli enterotoxin-induced enterosorption and its effects on intestinal mucosa cyclic 3′, 5′-AMP levels.Infection & Immunity 11:1038–1044
Lonroth, I., Holmgren, J., Lange, S. 1977. Chlorpromazine inhibits cholera toxin-induced intestinal hypersection.Med. Biol. 55:126–129
Maenz, D.D., Forsyth, G.W. 1982. Ricinoleate and deoxycholate are calcium ionophores in jejunal brush border vesicles.J. Membrane Biol. 70:125–133
Maenz, D.D., Forsyth, G.W. 1984. Calcium ionophore activity of intestinal secretory compounds: Anin vitro porcine model for the effects of bile acids, hydroxy-fatty acids and dioctyl sulfosuccinate.Digestion 30:138–150
Maenz, D.D., Forsyth, G.W. 1986. Cholera toxin facilitates calcium transport in jejunal brush border vesicles.Can. J. Physiol. Pharmacol. 64:568–574
Michaylova, V., Ilkova, P. 1971. Photometric determination of micro amounts of calcium with arsenazo III.Anal. Chem. 53:194–198
Rebois, R.V., Beckner, S.K., Brady, R.O., Fishman, P.H. 1983. Mechanism of action of glycopeptide hormones and cholera toxin: What is the role of ADP-ribosylation?Proc. Natl. Acad. Sci. USA 80:1275–1279
Tosteson, M.T., Tosteson, D.C. 1978. Bilayers containing ganglioside develop channels when exposed to cholera toxin.Nature (London) 275:142–144
Tsien, R.Y., Pozzan, T., Rink, T.J. 1982. Calcium homeostasis in intact lymphocytes: Cytoplasmic free calcium monitored with a new intracellularly trapped fluorescent indicator.J. Cell Biol. 94:325–334
Weiser, M.M. 1973. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation.J. Biol. Chem. 248:2536–2541
Weissman, G., Anderson, P., Serhan, C., Samuelsson, E., Goodman, E. 1980. A general method, employing arsenazo III in liposomes, for study of calcium ionophores: Results with A23187 and prostaglandins.Proc. Natl. Acad. Sci. USA 77:1506–1510
Wisnieski, B.J., Bramhall, J.S. 1981. Photolabelling of cholera toxin subunits during membrane penetration.Nature (London) 289:319–321
Yamamoto, T., Nakazawa, T., Miyata, T., Kaji, A., Yokota, T. 1984. Evolution and structure of two ADP-ribosylation enterotoxins,Escherichia coli heat-labile toxin and cholera toxin.FEBS Lett. 169:241–246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maenz, D.D., Gabriel, S.E. & Forsyth, G.W. Calcium transport affinity, ion competition and cholera toxin effects on cytosolic Ca concentration. J. Membrain Biol. 96, 243–249 (1987). https://doi.org/10.1007/BF01869306
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869306