Summary
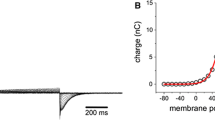
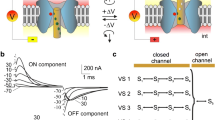
The voltage-dependent channel formed in planar lipid bilayers by colicin E1, or its channel-forming C-terminal fragments, is susceptible to destruction by the nonspecific protease pepsin under well-defined conditions. In particular, pepsin acts only from thecis side (the side to which colicin has been added) and only upon channels in the closed state. Channels in the open state are refractory to destruction bycis pepsin, and neither open nor closed channels are destroyed bytrans pepsin. Colicin E1 channels are normally turned on bycis positive voltages and turned off bycis negative voltages. For large (>80 mV) positive voltages, however, channels inactivate subsequent to opening. Associated with the inactivated state, some channels become capable of being turned on bycis negative voltages and turned off bycis positive voltages, as if the channel-forming region of the molecule has been translocated across the membrane. Consistent with this interpretation is the ability now oftrans pepsin to destroy these “reversed” channels when they are closed, but not when they are open, whereascis pepsin has no effect on them in either the open or closed state. Our results indicate that voltage gating of the E1 channel involves translocation of parts of the protein across the membrane, exposing different domains to thecis andtrans solutions in the different channel states.
Similar content being viewed by others
References
Cleveland, M.vB., Slatin, S., Finkelstein, A., Levinthal, C. 1983. Structure-function relations for a voltage-dependent ion channel: Properties of COOH-terminal fragments of colicin E1.Proc. Natl. Acad. Sci. USA 80:3706–3710
Cramer, W.A., Dankert, J.R., Uratani, Y. 1983. The membrane channel-forming bacteriocidal protein, colicin E1.Biochim. Biophys. Acta 737:173–193
Greenfield, L., Bjorn, M.J., Horn, G., Fong, D., Buck, G.A., Collier, R.J., Kaplan, D.A. 1983. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage β.Proc. Natl. Acad. Sci. USA 80:6853–6857
Heyer, E.J., Muller, R.U., Finkelstein, A. 1976. Inactivation of monazomycin-induced voltage-dependent conductance in thin lipid membranes: II. Inactivation produced by monazomycin transport through the membrane.J. Gen. Physiol. 67:731–748
Kagawa, Y., Racker, E. 1971. Partial resolution of the enzymes catalyzing oxidative phosphorylation: XXV. Reconstitution of particles catalyzing32Pi-adenosine triphosphate exchange.J. Biol. Chem. 246:5477–5487
Montal, M. 1974. Formation of bimolecular membranes from lipid monolayers.Methods Enzymol. 32:545–554
Morlon, J., Lloubes, R., Varenne, S., Chartier, M., Lazdunski, C. 1983. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate.J. Mol. Biol. 170:271–285
Mueller, P., Rudin, D.O., Tien, H.T., Wescott, W.C. 1963. Methods for the formation of single bimolecular lipid membranes in aqueous solution.J. Phys. Chem. 67:534–535
Noda, M., Shimizu, S., Tanabe, T., Takai, T., Kayano, T., Ikeda, T., Takahashi, H., Nakayama, H., Minamino, N., Kangawa, K., Matsuo, H., Raftery, M.A., Hirose, T., Inayama, S., Hayashida, H., Miyata, T., Numa, S. 1984. Primary structure ofElectrophorus electricus sodium channel deduced from cDNA sequence.Nature (London) 312:121–127
Raymond, L., Slatin, S.L., Finkelstein, A., Liu, Q.-R., Levinthal, C. 1985. Channels formed by colicin E1 in planar lipid bilayers are large and exhibit pH-dependent ion selectivity.J. Membrane Biol. 84:173–181
Raymond, L., Slatin, S.L., Finkelstein, A. 1986. Gating of a voltage-dependent channel (colicin E1) in planar lipid bilayers: Translocation of regions outside the channel-forming domain.J. Membrane Biol. 92:255–268
Schein, S.J., Kagan, B.L., Finkelstein, A. 1978. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes.Nature (London) 276:159–163
Slatin, S.L., Raymond, L., Finkelstein, A. 1985. Inactivation of a voltage-dependent channel (colicin E1): The role of membrane translocation of protein.Biophys. J. 47:430a
Varley, J.M., Boulnois, G.J. 1984. Analysis of a cloned colicin Ib gene: Complete nucleotide sequence and implications for regulation of expression.Nucleic Acids Res. 12:6727–6739
Wickner, W.T., Lodish, H.F. 1985. Multiple mechanisms of protein insertion into and across membranes.Science 230:400–407
Yamada, M., Ebina, Y., Miyata, T., Nakazawa, T., Nakazawa, A. 1982. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein.Proc. Natl. Acad. Sci. USA 79:2827–2831
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slatin, S.L., Raymond, L. & Finkelstein, A. Gating of a voltage-dependent channel (colicin E1) in planar lipid bilayers: the role of protein translocation. J. Membrain Biol. 92, 247–254 (1986). https://doi.org/10.1007/BF01869393
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869393