Abstract
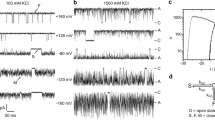
M2, an integral membrane protein of influenza A virus, was purified from either influenza A virus-infected CV-1 cells or from Spodoptera frugiperda (Sf9) cells infected with a recombinant-M2 baculovirus. The purified protein, when incorporated into phospholipid bilayer membranes, produced ion-permeable channels with the following characteristics: (1) The channels appeared in bursts during which unit conductances of diverse magnitudes (25–500 pS) were observed. (2) The most probable open state was usually the lowest unit conductance (25–90 pS). (3) The channels were selective for cations; t Na = 0.75 when 150 mm NaCl bathed both sides of the membrane. (4) Amantadine reduced the probability of opening of the high conductance state and also the conductance of the most probable state. (5) Reducing pH increased the mean current through the open channel as well as the conductance of the most probable state. (6) The sequence of selectivity for group IA monovalent cations was Rb > K > Cs ∼ Na > Li. The pH activation, amantadine block and ion selectivity of the M2 protein ion channel in bilayers are consistent with those observed on expression of the M2 protein in oocytes of Xenopus laevis as well as for those predicted for the proposed role of an ion channel in the uncoating process of influenza virus. The finding that the M2 protein has intrinsic ion channel activity supports the hypothesis that it has ion channel activity in the influenza virus particle.
Similar content being viewed by others
References
Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., eds. 1993. Current Protocols in Molecular Biology. Vols. 1 and 2. Wiley and Sons, NY
Black, R.A., Rota, P.A., Gorodkova, N., Cramer, A., Klenk, H.-D., Kendal, A.P. 1993. Production of the M2 protein of influenza A virus in insect cells is enhanced in the presence of amantadine. J. Gen. Virol. 74:1673–1677
Bron, R., Kendal, A.P., Klenk, H.-D., Wilschut, J. 1993. Role of the M2 protein in influenza virus membrane fusion: effects of amantadine and monensin on fusion kinetics. Virology 195:808–811
Bukrinskaya, A.G., Vorkunova, N.K., Kornilayeva, G.V., Narmanbetova, R.A., Vorkunova, G.K. 1982. Influenza virus uncoating in infected cells and effects of rimantadine. J. Gen. Virol. 60:49–59
Ciampor, F., Bayley, P.M., Nermut, M.V., Hirst, E.M., Sugrue, R.J., Hay, A. 1992a. Evidence that the amantadine-induced, M2-mediated conversion of influenza A virus hemagglutinin to the low pH conformation occurs in an acidic trans Golgi compartment. Virology 188:14–24
Ciampor, F., Thompson, C.A., Grambas, S., Hay, A.J. 1992b. Regulation of pH by the M2 protein of influenza A viruses. Virus Res. 22:247–258
Grambas, S., Bennett, M.S., Hay, A.J. 1992. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology 191:541–549
Grambas, S., Hay, A.J. 1992. Maturation of influenza A virus hemagglutinin-estimates of the pH encountered during transport and its regulation by the M2 protein. Virology 190:11–18
Duff, K.C., Ashley, R.H. 1992. The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers. Virology 190:485–489
Eisenberg, M., Hall, J.E., Mead, C.E. 1973. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J. Membrane Biol. 14:143–176
Eisenman, G., Horn, R. 1983. Ionic selectivity revisited: The role of kinetic and equilibrium processes in ion permeation through channels. J. Membrane Biol. 76:197–225
Eisenman, G., Sandblom, J.P. 1984. Modeling the gramicidin channel. Interpretation of experimental data using rate theory. Biophys. J. 45:88–90
Goldman, D. 1943. Potential, impedance and rectification in membranes. J. Gen. Physiol. 27:37–60
Hay, A.J. 1992. The action of adamantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Sem. Virol. 3:21–30
Hay, A.J., Wolstenholme, A., Skehel, J.J., Smith, M.H. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4:3021–3024
Helenius, A. 1992. Unpacking the incoming influenza virus. Cell 69:577–578
Hille, B. 1992. Ionic channels of excitable membranes. Sinauer Associates, Sunderland, MA
Hodgkin, A.L., Katz, B. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 108:37–77
Holsinger, L.J., Lamb, R.A. 1991. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology 183:32–43
Holsinger, L.J., Nichani, D., Pinto, L.H., Lamb, R.A. 1994. Influenza A virus M2 protein: a structure-function analysis. J. Virol. 68:1551–1563
Hunter, M., Giebisch, G. 1987. Multi-barrelled K channels in renal tubules. Nature 327:522–524
Krouse, M.E., Schneider, G.T., Gage, P.W. 1986. A large anion channel has seven conductance levels. Nature 319:58–60
Lamb, R.A. 1989. Genes and proteins of the influenza viruses. In: The Influenza Viruses. (R.M. Krug, editor), pp. 1–87. Plenum Press, NY
Lamb, R.A., Choppin, P.W. 1979. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc. Natl. Acad. Sci. USA 76:4908–4912
Lamb, R.A., Etkind, P.R., Choppin, P.W. 1978. Evidence for a ninth influenza viral polypeptide. Virology 91:60–78
Lamb, R.A., Lai, C.-J., Choppin, P.W. 1981. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc. Natl. Acad. Sci. USA 78:4170–4174
Lamb, R.A., Zebedee, S.L., Richardson, C.D. 1985. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 40:627–633
Marsh, M. 1992. Keeping the viral coat on. Curr. Biol. 2:379–381
Martin, K., and Helenius, A. 1991. Nuclear transport of influenza virus ribonucleoproteins: The viral matrix protein (M1) promotes export and inhibits import. Cell 67:117–130
Miller, C. 1982. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. (Land.) Ser. B 299:401–411
Neher, E., Steinback, J.H. 1978. Local anesthetics transiently block currents through single acetylcholine-receptor channels. J. Physiol. 277:153–176
Panayotov, P.P., Schlesinger, R.W. 1992. Oligomeric organization and strain-specific proteolytic modification of the virion M2 protein of influenza A H1N1 viruses. Virology 186:352–355
Pinto, L.H., Holsinger, L.J., Lamb, R.A. 1992. Influenza virus M2 protein has ion channel activity. Cell 69:517–528
Skehel, J.J. 1992. Influenza virus. Amantadine blocks the channel [news]. Nature 358:110–111
Skehel, J.J., Hay, A.J., Armstrong, J.A. 1978. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J. Gen. Virol. 38:97–110
Sugrue, R.J., Bahadur, G., Zambon, M.C., Hall-Smith, M., Douglas, A.R., Hay, A.J. 1990. Specific structural alteration of the influenza hemagglutinin by amantadine. EMBO J. 9:3469–3476
Sugrue, R.J., Hay, A.J. 1991. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology 180:617–624
Tosteson, M.T., Nibert, M.L., Fields, B.N. 1993. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc. Natl. Acad. Sci. USA 90:10549–10552
Wang, C., Takeuchi, K., Pinto, L.H., Lamb, R.A. 1993. The ion channel activity of the influenza A virus M2 protein: characterization of the amantadine block. J. Virol. 67:5585–5594
Wharton, S.A., Belshe, R.B., Skehel, J.J., Hay, A.J. 1994. Role of virion M2 protein in influenza virus uncoating: specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J. Gen. Virol. 75:945–948
Wiley, D.C., Skehel, J.J. 1987. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 56:365–394
Zebedee, S.L., Lamb, R.A. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 62:2762–2772
Zebedee, S.L., Richardson, C.D., Lamb, R.A. 1985. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J. Virol. 56:502–511
Author information
Authors and Affiliations
Additional information
The authors would like to acknowledge very helpful comments on the manuscript by Drs. Daniel C. Tosteson, Jose A. Halperin, John Collier and Roderick MacKinnon.
Research in the authors' laboratories was supported by Public Health Service research grants AI-20201 (R.A.L) and AI-31882 (L.H.P) from the National Institute of Allergy and Infectious Diseases. R.A.L. is an Investigator of the Howard Hughes Medical Institute.
Rights and permissions
About this article
Cite this article
Tosteson, M.T., Pinto, L.H., Holsinger, L.J. et al. Reconstitution of the influenza virus M2 ion channel in lipid bilayers. J. Membarin Biol. 142, 117–126 (1994). https://doi.org/10.1007/BF00233389
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233389