Abstract
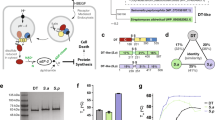
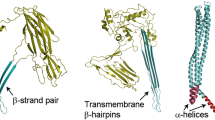
Diphtheria Toxin (DT) is a 535 amino acid exotoxin, whose active form consists of two polypeptide chains linked by an interchain disulphide bond. DT's N-terminal A fragment kills cells by enzymatically inactivating their protein synthetic machinery; its C terminal B chain is required for the binding of toxin to sensitive cells and for the translocation of the A fragment into the cytosol. This B fragment, consisting of its N-terminal T domain (amino acids 191–386) and its C-terminal R domain (amino acids 387–535) is responsible for the ion-conducting channels formed by DT in lipid bilayers and cellular plasma membranes. To further delineate the channel-forming region of DT, we studied channels formed by deletion mutants of DT in lipid bilayer membranes under several pH conditions. Channels formed by mutants containing only the T domain (i.e., lacking the A fragment and/or the R domain), as well as those formed by mutants replacing the R domain with Interleukin-2 (Il–2), have single channel conductances and selectivities essentially identical to those of channels formed by wild-type DT. Furthermore, deleting the N-terminal 118 amino acids of the T domain also has minimal effect on the single channel conductance and selectivity of the mutant channels. Together, these data identify a 61 amino acid stretch of the T domain, corresponding to the region which includes α-helices TH8 and TH9 in the crystal structure of DT, as the channel-forming region of the toxin.
Similar content being viewed by others
References
Cabiaux, V., Brasseur, R., Wattiez, R., Falmagne, P., Ruysschaert, J.M., Goormaghtigh, E. 1989. Secondary structure of diphtheria toxin and its fragments interacting with acidic liposomes studied by polarized infrared spectroscopy. J. Biol. Chem. 264:4928–4938
Cabiaux, V., Mindell, J.A., Collier, R.J. 1993. Involvement of Isoleucine 364 in the translocation of diphtheria toxin: relationship between cytotoxicity and pore formation. Infection and Immunity 61:2200–2202
Choe, S., Bennett, M.J., Fujii, G., Curmi, P.M., Kantardjieff, K.A., Collier, R.J., Eisenberg, D. 1992. The crystal structure of diphtheria toxin. Nature 357:216–222
Collier, R.J. 1990. Diphtheria toxin: Structure and function of a cytocidal protein. In: ADP-Ribosylating Toxins and G-Proteins: Insights into Signal Transduction. J. Moss and M. Vaughn, editors, pp. 3–19. Am. Soc. Microbiol., Washington, DC
Deleers, M., Beugnier, N., Falmagne, P., Cabiaux, V., Ruysschaert, J.M. 1983. Localization in diphtheria toxin fragment B of a region that induces pore formation in planar lipid bilayers at low pH. FEBS Lett. 160:82–86
Draper, R.K., Simon, M.I. 1980. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J. Cell Biol. 87:849–854
Falnes, P.O., Madshus, I.H., Sandvig, K., Olsnes, S. 1992. Replacement of negative by positive charges in the presumed membraneinserted part of diphtheria toxin B fragment. Effect on membrane translocation and on formation of cation channels. J. Biol. Chem. 267:12284–12290
Hoch, D.H. 1985. Botulinum, Tetanus, and Diphtheria Toxin Channels in Planar Lipid Bilayers. Ph.D. Thesis. Albert Einstein College of Medicine, Yeshiva University, Bronx, NY
Hoch, D.H., Finkelstein, A. 1985. Gating of large toxin channels by pH. Ann. NY Acad. Sci. 456:33–35
Hoch, D.H., Romero-Mira, M., Ehrlich, B.E., Finkelstein, A., DasGupta, B.R., Simpson, L.L. 1985 Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. USA 82:1692–1696
Kagan, B.L., Finkelstein, A., Colombini, M. 1981. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc. Natl. Acad. Sci. USA 78:4950–4954
Kagawa, Y., Racker, E. 1971. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicles catalyzing 32Pi-adenosine triphosphate exchange. J. Biol. Chem. 246:5477–5487
Kayser, G., Lambotte, P., Falmagne, P., Capiau, C., Zanen, J., Ruysschaert, J.M. 1981. A CNBR peptide located in the middle region of diphtheria toxin fragment B induces conductance change in lipid bilayers. Possible role of an amphipathic helical segment. Biochem. Biophys. Res. Commun. 99:358–363
London, E. 1992. Diphtheria toxin: membrane interaction and membrane translocation. Biochim. Biophys. Acta 1113:25–51
Madshus, I.H., Stenmark, H. 1992. Entry of ADP-ribosylating toxins into cells. Curr. Top. Microbiol. Immunol. 175:1–26
Mindell, J.A., Silverman, J.A., Collier, R.J., Finkelstein, A. 1992. Locating a residue in the diphtheria toxin channel. Biophys. J. 62:41–44
Mindell, J.A., Silverman, J.A., Collier, R.J., Finkelstein, A. 1994a. Structure-function relationships in the diphtheria toxin channel: II. A residue responsible for the channel's dependence on trans pH. J. Membrane Biol. 137:29–44
Mindell, J.A., Silverman, J.A., Collier, R.J., Finkelstein, A. 1994b. Structure-function relationships in the diphtheria toxin channel: III. Residues which affect the cis pH dependence of channel conductance. J. Membrane Biol. 137:45–57
Misler, S. 1983. Gating of ion channels made by a diphtheria toxin fragment in phospholipid bilayer membranes. Proc. Natl. Acad. Sci. USA 80:4320–4324
Montal, M. 1974. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 32:545–554
O'Keefe, D.O., Cabiaux, V., Choe, S., Eisenberg, D., Collier, R.J. 1992. pH-dependent insertion of proteins into membranes: Bchain mutation of diphtheria toxin that inhibits membrane translocation, Glu-349---Lys. Proc. Natl. Acad. Sci. USA 89:6202–6206
Papini, E., Sandona, D., Rappuoli, R., Montecucco, C. 1988. On the membrane translocation of diphtheria toxin: at low pH the toxin induces ion channels on cells. EMBO J. 7:3353–3359
Pappenheimer, A.M., Jr., Uchida, T., Harper, A.A. 1972. An immunological study of the diphtheria toxin molecule. Immunochemistry 9:891–906
Riggs, P. 1990. Expression and purification of maltose binding protein fusions. In: Current Protocols in Molecular Biology. Ausbel et al., editors. Wiley Interscience, Cambridge, MA
Sandvig, K., Olsnes, S. 1980. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 87:828–832
Sandvig, K., Olsnes, S. 1988. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J. Biol. Chem. 263:12352–12359
Tweten, R.K., Barbieri, J.T., Collier, R.J. 1985. Diphtheria toxin. Effect of substituting aspartic acid for glutamic acid 148 on ADP-ribosyltransferase activity. J. Biol. Chem. 260:10392–10394
Uchida, T., Pappenheimer, A.M., Jr., Greany, R. 1973. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J. Biol. Chem. 248:3838–3844
Uchida, T., Pappenheimer, A.M., Jr., Harper, A.A. 1972. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science 175:901–903
vanderSpek, J.C., Mindell, J.A., Finkelstein, A., Murphy, J.R. 1993. Structure/function analysis of the transmembrane domain of DAB(389)-Il-2, an interleukin 2 receptor-targeted fusion toxin. J. Biol. Chem. 268:12077–12082
Williams, D.P., Snider, C.E., Strom, T.B., Murphy, J.R. 1990. Structure/function analysis of interleukin-2-toxin (DAB486-IL-2). Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J. Biol. Chem. 265:11885–11889
Wonderlin, W.F., Finkel, A., French, R.J. 1990. Optimizing planar lipid bilayer single-channel recordings for high time resolution. Biophys. J. 58:289–297
Author information
Authors and Affiliations
Additional information
This work was supported by NIH grants AI22021, AI22848 (R.J.C.), T32 GM07288 (J.A.M.) and GM29210 (A.F.).
Rights and permissions
About this article
Cite this article
Silverman, J.A., Mindell, J.A., Zhan, H. et al. Structure-function relationships in diphtheria toxin channels: I. Determining a minimal channel-forming domain. J. Membarin Biol. 137, 17–28 (1994). https://doi.org/10.1007/BF00234995
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234995