Summary
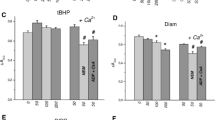
The effect of lowering intracellular pH on the membrane potential (E m ) of rat thymic lymphocytes was studied using the potential-sensitive dyebis-oxonol. Cells were acid loaded by addition of the electroneutral K+/H+ exchanging ionophore nigericin. Acidification to pH 6.3 in Na+-free solution resulted in a biphasic change inE m : an early transient hyperpolarization followed by a sustained depolarization. These changes were associated with a rise in cytosolic free Ca2+ ([Ca2+] i ). The hyperpolarization was eliminated when the change in [Ca2+] i was prevented using BAPTA, an intracellular Ca2+ chelator. Moreover, a similar hyperpolarization was elicited by elevation of [Ca2+] i at physiological pH i using ionomycin, suggesting involvement of Ca2+-activated K+ channels. In contrast, the depolarization phase could not be mimicked by raising [Ca2+] i with ionomycin. However, intracellular BAPTA effectively inhibited the acidificationinduced depolarization. Inhibition was also obtained by extracellular addition of EGTA or dithiothreitol, even when the external free Ca2+ concentration remained unaltered. These observations suggested a possible role of contaminating trace metals. Cytosolic acidification is envisaged to induce intracellular accumulation of one or more trace metals, which induces the observed changes inE m . Accordingly, similar changes inE m can be induced without acidification by the addition of small amounts of Cu2+ to the medium. The ionic basis of theE m changes induced by acidification and the significance of these observations are discussed.
Similar content being viewed by others
References
Blinks, J.R., Wier, W.G., Hess, P., Prendergast, F.G. 1982 Measurement of Ca2+ concentrations in living cellsProg. Biophys. Mol. Biol. 40:1–114
Boron, W.F., Boulpaep, E.L. 1983. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO −3 transport.J. Gen. Physiol. 81:53–94
Busa, W.B., Nuccitelli, R. 1984. Metabolic regulation via intracellular pH.Am. J. Physiol. 246:R409-R438
Deutsch, C., Lee, S.C. 1989. Modulation of K+ currents in human lymphocytes by pH.J. Physiol. (London) 413:399–413
Frelin, C., Vigne, P., Ladoux, A., Lazdunski, M. 1988. The regulation of the intracellular pH in cells from vertebrates.Eur. J. Biochem. 48:403–413
Golowasch, J., Kirkwood, A., Miller, C. 1986. Allosteric effects of Mg2+ on the gating of Ca2+-activated K− channels from mammalian skeletal muscle.J. Exp. Biol. 124:5–13
Grinstein, S., Cohen, S., Rothstein, A. 1984a. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport.J. Gen. Physiol. 83:341–369
Grinstein, S., Goetz, J.D., Rothstein, A. 1984b.22Na+ fluxes through thymic lymphocytes: I. Na+/Na+ and Na+/H+ exchange through an amiloride-sensitive pathway.J. Gen. Physiol. 84:565–584
Grinstein, S., Goetz, J.D., Rothstein, A. 1984c.22Na+ fluxes in thymic lymphocytes: II. Amiloride-sensitive Na+/H+ exchange pathway; reversibility of transport and asymmetry of the modifier site.J. Gen. Physiol. 84:585–600
Grinstein, S., Smith, J.D. 1989. Ca2+ induced charybdotoxinsensitive membrane potential changes in rat lymphocytes.Am. J. Physiol. 26:C197-C206
Grynkiewicz, G., Poenie, M., Tsien, R.Y. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties.J. Biol. Chem. 260:3440–3450
Kaila, K., Voipio, J. 1987. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance.Nature (London) 330:163–165
MacDougall, S.L., Grinstein, S., Gelfand E.W. 1988. Activation of Ca2+-dependent K+ channels in human B-lymphocytes by anti-immunoglobulin.J. Clin. Invest. 81:449–454
Mahaut-Smith, M.P., Schlichter, L.C., 1989. Ca2+-activated K+ channels in human B lymphocytes and rat thymocytes.J. Physiol. (London).415:69–83
Martell, A.E., Smith, R.M. 1974.Critical Stability Constants. Vol. 1. Plenum, New York
Martell, A.E., Smith, R.M. 1987Critical Stability Constants. Vol. 6. Plenum, New York
Moody, W. Jr. 1984. Effects of intracellular H+ on the electrical properties of excitable cells.Annu. Rev. Neurosci. 7:257–278
Oberhauser, A., Alvarez, O., Latorre, R. 1988. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane.J. Gen. Physiol. 92:67–86
Rink, T.J., Montecucco, C., Hesketh, T.R., Tsien, R.Y. 1980. Lymphocyte membrane potential assessed with fluorescent probes.Biochim. Biophys. Acta.595:65–70
Rink, T.J., Tsien, R.Y., Pozzan, T. 1982. Cytoplasmic pH and free Mg2+ in lymphocytes.J. Cell Biol. 95:189–196
Roos, A., Boron, W.F. 1981. Intracellular pH.Physiol. Rev. 61:296–434
Rudnick, G. 1986. ATP-driven H+ pumping into intracellular organelles.Annu. Rev. Physiol. 48:403–413
Thomas, R.C., 1984. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery.J. Physiol. (London) 354:3P-22P
Thomas, R.C., Meech, R.W. 1982. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones.Nature (London) 299:826–828
Tsien, R.Y., Pozzan, T., Rink, T.J. 1982. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes.Nature (London) 295:68–70
Tsein, R.Y., Rink, T.J. 1980. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium.Biochim. Biophys. Acta 599:623–638
Weinreich, D., Wonderlin, W.F. 1987. Copper activates a unique inward current in molluscan neurones.J. Physiol. (London) 394:429–443
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mason, M.J., Grinstein, S. Effect of cytoplasmic acidification on the membrane potential of T-lymphocytes: Role of trace metals. J. Membrain Biol. 116, 139–148 (1990). https://doi.org/10.1007/BF01868672
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01868672