Abstract
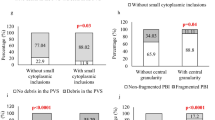
A study was made of the effects of low power laser irradiation on in vitro oocyte maturation rates and subsequent fertilization of immature bovine oocytes. Immature bovine oocytes from cows sacrificed at a slaughterhouse were irradiated with Helium-Neon laser (HeNe laser) irradiation at an energy density of 0.4 and 2.0 J cm−2. An oocyte group was left untreated, serving as control group. All oocytes were matured and fertilized in TCM-199 medium supplemented with 20% fetal calf serum (FCS). Maturation and fertilization rates obtained in the irradiated oocytes group were lower (p<0.001 andp<0.05, respectively) than those of the control group. Furthermore, the laser-treated oocytes showed important degenerative changes on both cytoplasm and chromosomes in comparison with untreated control oocytes which showed a homogenous cytoplasm and disperse chromosomes. It is concluded that the application of HeNe laser irradiation at 0.4 and 2.0 J cm−2 energy densities has a detrimental effect on in vitro maturation and fertilization process of immature bovine oocytes.
Similar content being viewed by others
References
Biggers JD. In: Biggers JO, Schuetz W (eds)Oogenesis, Baltimore, Maryland: Univ. Park Press, 1972:241–52
McGaughey RW. A comparison of the fluids from small and large ovarian follicle of the pig.Biol Reprod 1975,13(2):147–53
Wasserman P. The mammalian ovum. In: Knobil E, Neill J (eds)The Physiology of Reproduction, Vol 1. New York: Raven Press, 1988:69–102
Warnes GM, Moor RM, Johnson MH. Changes in protein synthesis during maturation of sheep oocytes in vivo and in vitro.J Reprod Fertil 1977,49(2):331–5
Caspers K. Laser stimulation therapy.Physics and Medical Rehabilitation 1977,18(9):426–45
Kana J, Hana D, Waidelich W. Effect of low power density radiation on healing of open skin wounds in rats.Arch Surg 1981,116:291–6
Korvacs L. The stimulatory effect of laser on the physiological healing of portio surface.Lasers Surg Med 1981,1:241–52
Ribari O. The stimulating effect of low power laser rays: Experimental examinations in otorhinolaryngology.Reviews in Laryngology 1981,102:531–3
Passarella S, Casamassima E, Molinari S et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by HeNe laser.FEBS Lett 1984,175:95–9
Yamada K. Biological effects of low power laser irradiation on clonal osteoblastic cells (MC3T3-E1).Nippon Seikeigeka Gakki Zasshi 1991,65:787–99
Tominaga R. Effects of HeNe laser irradiation on fibroblasts derived from scar tissue of rat palatal mucosa (Jpn).Kokubyo Gakki Zasshi 1990,57:580–5
Nara Y, Tsukamoto Y, Fukutani S et al. Stimulative effect of HeNe laser irradiation on cultured fibroblasts derived from human dental pulp.Lasers Life Sci 1992,4(4):249–52
Herbert KE, Bhusate LL, Scott DL et al. Effect of laser light at 820 nm on adenosine nucleotide levels in human lymphocytes.Lasers Life Sci 1989,3(1):37
Karu TI, Kalendo GS, Letokhov VS. Comparison of the action of powerful pulses of ultrashort UV on the DNA replication and transcription functions in proliferating and resting HeLa cells (Rus).Radiobiologiia 1984,24:17–20
Karu TI, Smolyaninova N, Selenin A. Long-term and short-term responses of human lymphocytes to HeNe laser radiation.Lasers Life Sci 1991,4(3):167
Devlin TM.Textbook of Biochemistry—with Clinical Correlations, 2nd edn. New York: John Wiley & Sons, 1986:123
Calderhead RG. On the correct reporting of parameters and consistent use of terminology in reporting clinical and experimental LLLT procedures.Laser Ther 1990,3:2
Ball GD, Leibfried ML, Lenz RW et al. Bovine fertilization in vitro: A temperature sensitive process.J Anim Sci 1982, 336
Wheeland RG, Walker NPJ. Lasers 25 years later.Int J Dermatol 1986,25:209–16
Abergel P, Meeker A, Lam S et al. Control of connective tissue metabolism by lasers: Recent developments and future prospects.J Am Acad Dermatol 1984,11:1142–50
Trelles MA, Mayayo E. Bone fracture consolidates faster with low-power laser.Lasers Surg Med 1987,7:36–45
Goldman JA, Chiapella J, Casey H et al. Laser therapy of rheumatoid arthritis.Lasers Surg Med 1980,1:93–101
Ocaña Quero JM, Gomez Villamandos R, Moreno Millan M, Santisteban Valenzuela JM. The effect of the Helium-Neon laser radiation on the in vitro fertilization of bovine oocytes. Proceedings of the 11th European Coll. Cytogenet. Domest. Anim. 1994, 174–8
Bielanski A, Hare WCD. Development in vitro of bovine embryos after exposure to continuous helium-neon laser light.Theriogenology 1992,37:192
Hirao Y, Yanagimachi R. Detrimental effect of visible light on meiosis of mammalian eggs in vitro.J Exp Zool 1978,206:365–70
Basford JR. Low-energy laser therapy: Controversies and new research findings.Lasers Surg Med 1989,9:1–5
Karu TI.Photobiology of Low-Power Laser Therapy. Chur: Harwood Academy Publishers, 1989:205
Karu TI. Effects of visible radiation on cultured cells.Photochem Photobiol 1990,52(6):1089
Tiphlova O, Karu TI. Role of primary photoacceptors in low-power laser effects: Action of HeNe laser radiation on bacteriophage T4-Escherichia coli interaction.Lasers Surg Med 1989,9:67
Karu TI, Tiphlova O. Stimulation ofE. coli growth by laser and incoherent red light.II Nuovo Cimento 1983,2D(4):1138
Karu TI, Tiphlova O. Effect of irradiation with monochromatic visible light on cAMP content in Chinese hamster fibroblast.II Nuovo Cimento 1987,9D(10):1245
Cheng LY, Packer L. Photodamage to hepatocytes by visible light.FEBS Lett 1979,97:124
Parshad R, Sanford KK, Taylor WG et al. Effect of intensity and wavelength of fluorescent light on chromosome damage in cultured mouse cells.Photochem Photobiol 1979,29:971
Tyrrell RM, Pidoux M. Action spectra for human skin cells: Estimates of the relative cytotoxicity of the middle ultraviolet, near ultraviolet, and violet regions of sunlight on epidermal keratinocytes.Cancer Res 1987,47:1825
Loevschall H.Survey of Preliminary Low Level Laser Reports (Danish), Roskilde: Roskilde University, 1989:45
O'Kane S, Shields DT, Gilmore WS, Allen JM. Low intensity laser irradiation inhibits tritiated thymidine incorporation in the hemopoietic cell lines HL-60 and U937.Lasers Surg Med 1994,14:34–9
Olson JE, Schimmerling W, Gundy GC, Tobias CA. Laser microirradiation of cerebellar neurons in culture. Electrophysiological and morphological effects.Cell Biophys 1981,3:349
McKelvey VJ, Keegan AL, Allen JM. Induction of DNA damage by low level laser irradiation in Friend mouse erythroleukemia cells.Mutat Res 1992,271:131
Pardee AB, Dubrow R, Hamlin JL, Kletzien RF. Animal cell cycle.Ann Rev Biochem 1978,47:715–50
Lucke-Huhle C. Alpha-irradiation induced G2 delay. A period of cell recovery.Radiat Res 1982,89:298–308
Passarella S, Roncali L, Cicero R, Quagliariello E. New ultrastructural conformations of mitochondria irradiated in vitro with a helium-neon laser.Lasers Life Sci 1988,2(3):161–71
Lehninger AL. Mitochondria and calcium ion transport.Biochem J 1970 118:129–38
Bradley MP, Forrester IT. A sodium calcium exchange mechanism in plasma membrane vesicles isolated from ram flagella.FEBS Lett 1980,121:15–18
Babcock DF, Pfeiffer DR. Independent elevation of cytosolic Ca2+ and Ph of mammalian sperm by voltage dependent and pH sensitive mechanism.J Biol Chem 1987,262:15041–7
Sallet C, Passarella S, Quagliariello E. Effects of selective irradiation on mammalian mitochondria.Photochem Photobiol 1987,45:433–8
Darnell J, Lodish H, Baltimore D.Molecular Cell Biology, 2nd edn. New York: Scientific American Books, Inc., 1990:345
Alberts B, Bray D, Lewis J et al.Molecular Biology of the Cell, 2nd edn. New York & London: Garland Publishing Inc., 1989:67
Shepherd GM.Neurobiology, New York: Oxford University Press, 1983:123
Halliwell B, Gutteridge JMC.Free Radicals in Biology and Medicine, 2nd edn. Oxford: Oxford University Press, 1989:89
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Quero, J.M.O., Villamandos, R.J.G., Millan, M.M. et al. The effect of helium-neon laser irradiation on in vitro maturation and fertilization of immature bovine oocytes. Laser Med Sci 10, 113–119 (1995). https://doi.org/10.1007/BF02150848
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02150848