Summary
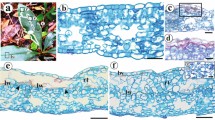
Cynodon dactylon (Poaceae) leaf pieces recovered from the frass of final-instar Paratrytone melane larvae (Lepidoptera: Hesperiidae) were composed of 14–22 percent crushed cells and 78–86 percent uncrushed cells, yet approximate digestibilities of soluble carbohydrates and protein averaged 78 and 88 percent, respectively. Therefore, nutrients from uncrushed cells were extracted by P. melane. The ability of P. melane and another leaf-snipping lepidopteran, Pseudaletia unipuncta (Noctuidae), to digest the contents of uncrushed bundle sheath and mesophyll cells in C. dactylon was examined with transmission electron microscopy. Organelles and plasma membranes were digested in the foreguts and midguts of both species. These findings suggest that nutrients in uncrushed leaf cells may be extracted through plasmodesmata and cell wall pores after membranes are digested. The generality of leaf-snipping, vis-a-vis leaf crushing, among larval Lepidoptera was assessed by surveying the mandible morphologies of 202 species. In 82 percent of the species surveyed only incisor regions were present. I conclude that leaf-snipping is a common mode of feeding among phytophagous Lepidoptera and that the digestion of cell contents is efficient despite the fact that few of the cells of ingested plant tissues are crushed.
Similar content being viewed by others
References
Barbehenn R (1989) The nutritional ecology and mechanisms of digestion of C3 and C4 grass-feeding Lepidoptera. PhD thesis, University of California, Berkeley
Baron-Epel O, Gharyal PK, Schindler M (1988) Pectins as mediators of wall porosity in soybean cells. Planta 175:389–395
Bennack DE (1981) The effects of mandible morphology and photosynthetic pathway on selective herbiory in grasshoppers. Oecologia 51:281–283
Berenbaum M (1980) Adaptive significance of midgut pH in larval lepidoptera. Am Nat 115:138–146
Bernays EA, Barbehenn RV (1987) Nutritional ecology of grass foliage-chewing insects. In: Slansky F Jr., Rodriguez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. John Wiley and Sons, New York, pp 147–176
Bernays EA, Janzen DH (1988) Saturniid and sphingid caterpillars: two ways to eat leaves. Ecology 69:1153–1160
Berry JA, Downton WJS, Tregunna EB (1970) The photosynthetic carbon metabolism of Zea mays and Gomphrena globosa: the location of CO2 fixation and the carboxyl transfer reactions. Can J Bot 48:777–786
Biedermann W (1919) Beiträge zur vergleichenden Physiologie der Verdauung. Arch ges Physiol 174:392–425
Bjorkman O, Berry J (1973) High-efficiency photosynthesis. Sci Am 229:80–93
Boys M (1981) Food selection by some graminivorous Acrididae. PhD thesis, University of Oxford, U.K.
Brown JW (1984) Host records for Paratrytone melane (Edwards) (Hesperiidae). J Lep Soc 38:138
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205:1144–1147
Caswell M, Reed FC (1975) Indigestibility of C4 bundle sheath cells by the grasshopper, Melanoplus confusus. Ann Entomol Soc Am 68:686–688
Caswell H, Reed FC (1976) Plant-herbivore interactions: the indigestibility of C4 bundle sheath cells by grasshoppers. Oecologia 26:151–156
Caswell H, Reed F, Stephenson SN, Werner PA(1973) Photosynthetic pathways and selective herbivory: a hypothesis. Am Nat 107:465–480
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37:49–57
Chapman RF (1982) The insects: structure and function. Harvard University Press, Cambridge
Chapman MS, Suh SW, Cascio D, Smith WW, Eisenberg D (1987) Sliding-layer conformational change limited by the quaternary structure of plant RuBisCO. Nature 329:354–356
Cockfield SD (1988) Relative availability of nitrogen in host plants of invertebrate herbivores: three possible nutritional and physiological definitions. Oecologia 77:91–94
Dow JAT (1986) Insect midgut function. Adv Insect Physiol 19:187–328
Evans AC (1939) The utilization of food by the larvae of the buff-tip Phalera bucephala (Linn.) (Lepidopt.) Proc Royal Entomol Soc (London) 14:25–30
Evert RF, Eschrich W, Heyser W (1977) Distribution and structure of the plasmodesmata in mesophyll and bundle sheath cells of Zea mays L. planta 136:77–89
Farrar SC, Farrar JF (1986) Compartmentation and fluxes of sucrose in intact leaf blades of barley. New Phytol 103:645–657
Freifelder D (1987) Molecular biology. Jones and Bartlett Publishers, Inc., Boston
Harvey GT (1975) Nutritional studies of eastern spruce budworm (Lepidoptera: Tortricidae) II. Starches. Can Entomol 107:717–728
Heidorn T, Joern A (1984) Differential herbivory on C3 versus C4 grasses by the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 65:19–25
Hocking B, Depner KR (1961) Larval nutrition in Agrotis orthogonia (Lepidoptera: Phalaenidae): digestive enzymes. Ann Entomol Soc Am 54:86–98
Horie Y, Nakasome S, Watanabe K, Nakamura M, Suda H (1985) Daily ingestion and utilization of various kinds of nutrients by the silkworm, Bombyx mori (Lepidoptera: Bombycidae). Appl Entomol Zool 20:159–172
Jones CG, Hare JD, Compton SJ (1989) Measuring plant protein with the Bradford assay. I. Evaluation and standard method. J Chem Ecol 15:979–992
Ku SB, Schmitt MR, Edwards GE (1979) Quantitative determination of RuBP carboxylase-oxygenase in leaves of several C3 and C4 plants. J Exp Bot 30:89–98
Martin MM, Martin JS (1984) Surfactants: their role in preventing the precipitation of protein by tannins in insect guts. Oecologia 61:342–345
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi Pty Ltd., Melbourne
Peterson A (1951) Larvae of insects. Part 1: Lepidoptera and plant infesting Hymenoptera. Edwards Bros., Ann Arbor
Rintamaki E (1989) Formation of disulphide cross-linked aggregates of large subunit from higher plant ribulose-1,5-bisphosphate carboxylase-oxygenase. J Exp Bot 40:1305–1313
Robards AW (1976) Plasmodesmata in higher plants. In: Gunning BES, RobardsAW (eds) Intercellular communication in plants: studies on plasmodesmata. Springer-Verlag, New York, pp 15–57
Rybicki M (1957) Mechanism of digestion of leaves of green plants by some lepidopterous caterpillars. Acta Biol Exp (Warsaw) 17:289–316
Santos CD, Ferreira C, Terra WR (1983) Consumption of food and spatial organization of digestion in the cassava hornworm, Erinnyis ello. J Insect Physiol 29:707–714
Santos CD, Terra WR (1986) Distribution and characterization of oligomeric digestive enzymes from Erinnyis ello larvae and inferences concerning secretory mechanisms and the permeability of the peritrophic membrane. Insect Biochem 16:691–700
Schultz JC, Lechowicz MJ(1986) Hostplant, larval age, and feeding behavior influence midgut pH in the gypsy moth (Lymantria dispar). Oecologia 71:133–137
Stehr FW (1987) Immature insects. Kendall/Hunt Publishing Co., Dubuque, Iowa
Terry BR, Robards AW (1987) Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 171:145–157
Thomson WW, Moeller CH (1983) Effects of TWEEN-20, polyoxyethylene sorbitan monolaurate on the ultrastructure and organization of chloroplast membranes. Protoplasma 114:173–178
Valle EM, Craig S, Hatch MD, Heldt HW (1989) Permeability and ultrastructure of bundle sheath cells isolated from C4 plants: structure-function studies and the role of plasmodesmata. Botanica Acta 102:276–282
Vonk HJ, Western JRH (1984) Comparative biochemistry and physiology of enzymatic digestion. Academic Press, New York
Wigglesworth VB (1972) The principles of insect physiology. Methuen and Co. London
Yemm EW and Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barbehenn, R.V. Digestion of uncrushed leaf tissues by leaf-snipping larval Lepidoptera. Oecologia 89, 229–235 (1992). https://doi.org/10.1007/BF00317222
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317222