Abstract
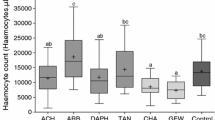
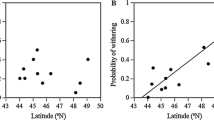
We tested the deme-formation hypothesis experimentally with four populations of leaf-galling grape phylloxera, Daktulosphaira vitifoliae, and its host, canyon grape (Vitis arizonica). An experiment designed to examine preference and performance showed that phylloxera populations did not significantly prefer their original host relative to other hosts in the percent available leaves galled. There were significant herbivore population effects (P<0.01), host effects (P<0.001), and population x host interaction effects (P<0.001). Herbivore populations had different colonizing abilities (performance, as measured in the mean number of galls per leaf) on an individual host (P<0.001), but there was no host effect. Host genotype significantly affected phylloxera performance, measured as survivorship (P<0.01), but a phylloxera population did not necessarily have higher survivorship on its original host. Differences in fecundity, an-other measurement of performance, were due to intrinsic differences among herbivore populations (P<0.05), and not related to host genotype. There was no correlation between distance from a phylloxera population in the field and a host's susceptibility to attack. There was a significant positive relationship between levels of infestation on a clone in the field and its susceptibility to colonization experimentally (P<0.05), suggesting inherent differences in host resistance and susceptibility. These results did not support the deme-formation hypothesis. In a second experiment, host clone x water treatment interactions affected phylloxera survivorship (P<0.05) and fecundity (P<0.05). We conclude that host genotype x environment interactions may prevent sessile, parthenogenetic herbivores from locally adapting to individual host genotypes.
Similar content being viewed by others
References
Alstad DN, Edmunds GF (1983) Adaptation, host specificity, and gene flow in the black pineleaf scale. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 413–426
Ayre DJ (1985) Localized adaptation of clones of the sea anemone Actinia tenebrosa. Evolution 39:1250–1260
Cates RG, Redak RA (1987) Variation in terpene chemistry of Douglas-fir and its relationship to western spruce budworm success. In: Spencer KC (ed) Chemical mediation of coevolution. Pergamon Press, Oxford
Cobb NS (1990) The role of host heterogeneity in preventing deme formation or individual trees, MS thesis, Northern Arizona University, Flagstaff
Cobb NS, Whitham TG (1993) Herbivore deme formation on individual trees: a test case. Oecologia 94:496–502
DeBenedictis JA, Granett J (1992) Variability of responses of grape phylloxera (Homoptera: Phylloxeridae) to bioassays that discriminate between California biotypes. J Econ Entomol 85: 1527–1534
Dewey SE, Heywood JS (1988) Spatial genetic structure in a population of Psycotria nervosa. 1. distribution of genotypes. Evolution 42:834–838
Edmunds GF, Alstad DN (1978) Coevolution in insect herbivores and conifers. Science 199:941–945
Edmunds GF, Alstad DN (1981) Responses of black pineleaf scales to host plant variability. In: Denno RF, Dingle H (eds) Insects and life history patterns: habitat and geographic variation. Springer, Berlin Heidelberg New York, pp 29–38
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, New York
Fong G, Walker MA, Granett J (1995) RAPD assessment of California phylloxera diversity. Mol Ecol 4:459–464
Gould F (1983) Genetics of plant-herbivore systems: interactions between applied and basic study. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 599–653
Granett J, Coheen AC, Lider LA (1987) Grape phylloxera in California. Calif Agric 41:10–12
Granett J, Timper P, Lider LA (1985) Grape phylloxera (Daktulosphaira vitifoliae) (Homoptera: Phylloxeridae) biotypes in California. J Econ Entomol 78:1463–1467
Halle F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests: an architectural analysis. Springer, Berlin Heidelberg New York
Jaenike J, Parker ED, Jr, Selander RK (1980) Clonal niche structure in the parthenogenetic earthworm Octolasion tyrtaeum. Am Nat 116:196–205
Karban R (1987) Effects of clonal variation of the host plant, interspecific competition, and climate on the population size of folivorous thrips. Oecologia 74:298–303
Karban R (1989) Fine-scale adaptation to herbivorous thrips to individual host plants. Nature 340:60–61
Kearsley MJC, Whitham TG (1989) Developmental changes in resistance to herbivory: implications for individuals and populations. Ecology 70:422–434
Kimberling DN, Scott ER, Price PW (1990) Testing a new hypothesis: plant vigor and phylloxera distribution on wild grape in Arizona. Oecologia 84:1–8
King PD, Rilling G (1985) Variations in the galling reaction of grapevines: evidence of different phylloxera biotypes and clonal reaction to phylloxera. Vitis 24:32–42
Lynch M (1984) Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q Rev Biol 59: 257–290
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
McPheron BA, Smith C, Berlocher SH (1988) Microgeographic genetic variation in the apple maggot Rhagoletis pomonella. Genetics 119:445–451
Mitter C, Futuyma DJ (1979) Population consequences of feeding habits in some forest lepidoptera. Genetics 92:1005–1021
Mopper S, Whitham TG, Price PW (1990) Plant phenotype and interspecific competition between insects determine sawfly performance and density. Ecology 71:2135–2144
Moran N (1981) Intraspecific variability in herbivore performance and host quality: a field study of Uroleucon caligatum (Homoptera: Aphididae) and its Solidago hosts (Asteraceae). Ecol Entomol 6:301–306
Moran N, Whitham TG (1988) Evolutionary reduction of complex life cycles: Loss of host-alternation in Pemphigus (Homoptera: Aphididae). Evolution 42:717–728
Moran N, Whitham TG (1990) Differential colonization of resistant and susceptible host plants: Pemphigus and Populus. Ecology 71:1059–1067
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton, NJ
Selander RK, Hudson RO (1976) Animal population structure under close inbreeding: the land snail Rumina in southern France. Am Nat 110:695–718
Service P (1984) Genotype interactions in an aphid-host plant relationship: Uroleucon rudbeckiae and Rudbeckia laciniata. Oecologia 61:271–276
Service PM, Lenski RE (1982) Aphid genotypes, plant phenotypes, and genetic diversity: a demographic analysis of experimental data. Evolution 36:1276–1282
Simms EL, Rausher MD (1989) The evolution of resistance to herbivory in Ipomoea purpurea. II. Natural selection by insects and costs of resistance. Evolution 43:573–585
Stevenson AB (1964) Seasonal life history of root infesting Phylloxera vitifoliae (Fitch) in Ontario. Can Entomol 96:979–987
Stevenson AB (1970) Strains of grape phylloxera in Ontario with different effects on the foliage of certain grape cultivars. J Econ Entomol 63:135–138
Unruh TR, Luck RF (1987) Deme formation in scale insects: a test with the pinyon needle scale and a review of other evidence. Ecol Entomol 12:439–449
Wainhouse D, Howell RS (1983) Intraspecific variation in beech scale populations and in susceptibility of their host Fagus sylvatica. Ecol Entomol 8:351–359
Wapshere AJ, Helm KF (1987) Phylloxera and Vitis: An experimentally testable coevolutionary hypothesis. Am J Enol Vitic 38:216–222
White TCR (1974) A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema sauvis in a plantation of Pinus radiata in New Zealand. Oecologia 16:279–301
Whitham TG (1981) Individual trees as heterogeneous environment: Adaptation to herbivory or epigenetic noise? In: Denno RF, Dingle H (eds) Insect life history patterns: habitat and geographic variation. Springer, Berlin Heidelberg, New York, pp 9–27
Whitham TG (1983) Host manipulation of parasites: within-plant variation as a defense against rapidly evolving pests. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 15–41
Whitham TG, Slobodchikoff CN (1981) Evolution by individuals plant-herbivore interactions, and mosaics of genetic variability: the adaptive significance of somatic mutations in plants. Oecologia 49:287–292
Whitham TG, Williams AG, Robinson AM (1984) The variation principle: Individual plants as temporal and spatial mosaics of resistance to rapidly evolving pests. In: Price PW, Slobodchikoff CN, Gaud WS (eds) A new ecology: novel approaches to interactive systems. Wiley, New York, pp 15–51
Williams RN, Shambaugh GF (1988) Grape phylloxera (Homoptera: Phylloxeridae) biotypes confirmed by electrophoresis and host susceptibility. Ann Entomol Soc Am 81:1–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimberling, D.N., Price, P.W. Variability in grape phylloxera preference and performance on canyon grape (Vitis arizonica). Oecologia 107, 553–559 (1996). https://doi.org/10.1007/BF00333948
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00333948