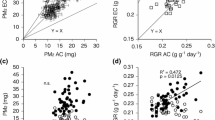
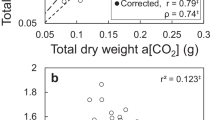
Abstract
In assessing the capacity of plants to adapt to rapidly changing global climate, we must elucidate the impacts of elevated carbon dioxide on reproduction, fitness and evolution. We investigated how elevated CO2 influenced reproduction and growth of plants exhibiting a range of floral morphologies, the implications of shifts in allocation for fitness in these species, and whether related taxa would show similar patterns of response. Three herbaceous, annual species each of the genera Polygonum, Ipomoea, and Cassia were grown under 350 or 700 ppm CO2. Vegetative growth and reproductive output were measured non-destructively throughout the full life span, and vegetative biomass was quantified for a subsample of plants in a harvest at first flowering. Viability and germination studies of seed progeny were conducted to characterize fitness precisely. Early vegetative growth was often enhanced in high-CO2 grown plants of Polygonum and Cassia (but not Ipomoea). However, early vegetative growth was not a strong predictor of subsequent reproduction. Phenology and production of floral buds, flowers, unripe and abscised fruits differed between CO2 treatments, and genera differed in their reproductive and fitness responses to elevated CO2. Polygonum and Cassia species showed accelerated, enhanced reproduction, while Ipomoea species generally declined in reproductive output in elevated CO2. Seed “quality” and fitness (in terms of viability and percentage germination) were not always directly correlated with quantity produced, indicating that output alone may not reliably indicate fitness or evolutionary potential. Species within genera typically responded more consistently to CO2 than unrelated species. Cluster analyses were performed separately on suites of vegetative and reproductive characters. Some species assorted within genera when these reproductive responses were considered, but vegetative responses did not reflect taxonomic affinity in these plants. Congeners may respond similarly in terms of reproductive output under global change, but fitness and prognoses of population persistence and evolutionary performance can be inferred only rarely from examination of vegetative characters alone.
Similar content being viewed by others
References
Aarssen LW, Taylor DR (1992) Fecundity allocation in herbaceous plants. Oikos 65: 225–232
Bazzaz FA, Garbutt K (1988) The response of annuals in competitive neighborhoods: effects of elevated CO2. Ecology 69: 937–946
Bazzaz FA, McConnaughay KDM (1992) Plant-plant interactions in elevated CO2 environments. Aust J Bot 40: 547–563
Bazzaz FA, Garbutt K, Reekie EG, Williams WE (1989) Using growth analysis to interpret competition between a C3 and a C4 annual under ambient and elevated CO2. Oecologia 79: 223–235
Bazzaz FA, Ackerly DD, Woodward FI, Rochefort L (1992) CO2 enrichment and dependence of reproduction on density in an annual plant and a simulation of its population dynamics. J Ecol 80: 643–651
Bradshaw AD, McNeilly T (1991) Evolutionary response to global climate change. Ann Bot 67: 15–22
Carter DR, Peterson KM (1983) Effects of a CO2-enriched atmosphere on the growth and competitive interaction of a C3 and a C4 grass. Oecologia 58: 188–193
Chiariello NR, Gulmon S (1991) Stress effects on plant reproduction. In: Mooney HA, Winner WE, Pell AJ (1991) Response of plants to multiple stresses. Academic Press, New York, pp 161–189
Curtis PS, Drake BG, Leadley PW, Arp WJ, Whigham DF (1989) Growth and senescence in plant communities exposed to elevated CO2 concentrations on an estuarine marsh. Oecologia 78: 20–26
Curtis PS, Snow AA, Miller AS (1994) Genotype-specific effects of elevated CO2 on fecundity in wild radish (Raphanus raphanistrum). Oecologia 97: 100–105
Fernald ML (1970) Gray's manual of botany, 8th edn. Van Nostrand Company, New York
Frazee JE, Marquis RJ (1994) Environmental contribution to floral trait variation in Chamaecrista fasciculata (Fabaceae: Caesalpinoideae). Am J Bot 81: 206–215
Garbutt K, Bazzaz FA (1984) The effects of elevated CO2 on plants. III. Flower, fruit and seed production and abortion. New Phytol 98: 433–446
Geber MA (1990) The cost of meristem limitation in Polygonum arenastrum: negative genetic correlations between fecundity and growth. Evolution 44: 799–819
Geber MA, Dawson TE (1992) Evolutionary responses of plants to global change. In: Karieva PM, Kingsolver JG, Huey RB (eds) Biotic interactions and global change. Sinauer Associates, Sunderland, Mass, pp 179–197
Gleason HA, Cronquist A (1991) Manual of vascular plants of Northeastern United States and adjacent Canada, 2nd edn. New York Botanical Garden, New York
Grime JP (1993) Vegetation functional classification systems as approaches to predicting and quantifying vegetation change. In: Solomon AM, Shugart HH (eds) Vegetation dynamics and global change. Chapman and Hall, New York, pp 293–305
Hammerton JL, Jalloq MC (1970) Studies on weed species of the genus Polygonum. VI. Environmental effects on seed weight, seed polymorphism and germination behavior in P. lapathifolium and P. persicaria. Weed Res 10: 204–217
Hardin IW, Doersken C, Hobson M, Herndon D, Thomas F (1972) Pollination ecology and floral biology of four weedy genera in southern Oklahoma. Southwest Nat 16: 403–412
Hesketh JD, Helmers H (1975) Floral initiation in four plant species growing in CO2 enriched air. Environ Control Biol 11: 51–53
Hunt R, Hand DW, Hannah MA, Neal AM (1991) Response to CO2 enrichment in 27 herbaceous species. Func Ecol 5: 410–421
Irwin HS, Barneby RC (1982) The American Cassiinae: a synoptical revision of Leguminosae tribe Cassieae subtribe Cassiinae in the New World. Mem NY Bot Gard 35: parts I and II
Kendall AC, Turner JC, Thomas SM (1985) Effects of CO2 enrichment at different irradiances on growth and yield of wheat I. Effects of cultivar and of duration of CO2 enrichment. J Exp Bot 36: 252–260
Kimball BA (1983) Carbon dioxide and agricultural yield: an assemblage and analysis of 430 prior observations. Agron J 75: 779–788
Lakon G (1948) The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiol 23: 389–393
Lee TD, Bazzaz FA (1982) Regulation of fruit and seed production in an annual legume, Cassia fasciculata. Ecology 63: 1363–1373
Lee TD, Bazzaz FA (1986) Maternal regulation of fecundity: nonrandom ovule abortion in Cassia fasciculata Michx. Oecologia 68: 459–465
Marc J, Gifford RM (1984) Floral initiation in wheat, sunflower and sorghum under carbon dioxide enrichment. Can J Bot 62: 9–14
Martin ME, Lee TD (1993) Self pollination and resource availability affect ovule abortion in Cassia fasciculata (Caesalpinaceae). Oecologia 94: 503–509
Phillips OL, Gentry AH (1994) Increasing turnover through time in tropical forests. Science 263: 954–958
Poorter H (1993) Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104/105: 77–97
Reekie EG, Bazzaz FA (1991) Effect of elevated CO2 on phenology and growth in four annual species grown as individuals and in competition. Can J Bot 69: 2475–2481
Reekie EG, Bazzaz FA (1992) Cost of reproduction as reduced growth in genotypes of two congeneric species with contrasting life histories. Oecologia 90: 21–26
Reekie JYC, Hicklenton PR, Reekie EG (1994) Effects of elevated CO2 on time of flowering in four short-day and four long-day species. Can J Bot 72: 533–538
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43: 223–225
Samson DA, Werk KS (1986) Size-dependent effects in the analysis of reproductive effort in plants. Am Nat 127: 667–680
Simmonds NW (1945) Biological flora of the British Isles: Polygonum persicaria L. J Ecol 33: 121–131
Smith TM, Shugart HH, Woodward FI, Burton PJ (1993) Plant functional types. In: Solomon AM, Shugart HH (eds) Vegetation dynamics and global change. Chapman and Hall, New York, pp 272–292
St. Omar L, Horvath SM (1983) Elevated carbon dioxide concentrations and whole plant senescence. Ecology 64: 1311–1314
Strain B, Cure JD (1985) Direct effects of increasing carbon dioxide on vegetation. DOE/ER-0238, Department of Energy, NTIS, Springfield, Va
Sultan SE, Bazzaz FA (1993) Phenotypic plasticity in Polygonum persicaria. I. Diversity and uniformity in genotypic norms of reaction to light. Evolution 47: 1009–1031
Thomas SC, Bazzaz FA (1993) The genetic component in plant size hierarchies: norms of reaction to density in a Polygonum species. Ecol Monogr 63: 231–249
United States Department of Agriculture (1971) Common weeds of the United States. Dover Publications, New York
Velleman PF (1992) Data Disk Handbook. Odesta Corporation, Northbrook, Illinois.
Watson MA (1984) Developmental constraints: effects on population growth and patterns of resource allocation in a clonal plant. Am Nat 123: 411–426
Wetherald RT (1991) Changes of temperature and hydrology caused by an increase of atmospheric carbon dioxide as predicted by general circulation models. In: Wyman RL (ed) Global climate and life on earth. Routledge, Chapman and Hall, New York, pp 1–17
Wigley TML, Raper SCB (1992) Implications for climate and sea level of revised IPCC emissions scenarios. Nature 357: 293–300
Wilkinson L, Hill A, Welna JP, Birkenbeuel GK (1992) SYSTAT for Windows: Statistics, Version 5. SYSTAT, INc., Evanston, Illinois
Wulff RD, Alexander HM (1985) Intraspecific variation in the response to CO2 enrichment in seeds and seedlings of Plantago lanceolata L. Oecologia 66: 458–460
Zangerl AR, Bazzaz FA (1983) Plasticity and genotypic variation in photosynthetic behavior of an early and a late successional species of Polygonum. Oecologia 57: 270–273
Zangerl AR, Bazzaz FA (1984) The response of plants to elevated CO2. II. Competitive interactions among annual plants under varying light and nutrients. Oecologia 62: 412–417
Ziska LH, Teramura AH (1992) Intraspecific variation in the response of rice (Oryza sativa) to increased CO2-photosynthetic, biomass and reproductive characteristics. Physiol Plant 84: 269–276
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Farnsworth, E.J., Bazzaz, F.A. Inter- and intra-generic differences in growth, reproduction, and fitness of nine herbaceous annual species grown in elevated CO2 environments. Oecologia 104, 454–466 (1995). https://doi.org/10.1007/BF00341343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00341343