Summary
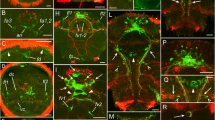
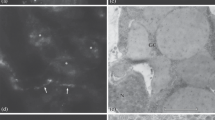
The complex catecholaminergic (CA) nervous system of the polychaete Ophryotrocha puerilis is documented using glyoxylic acid induced fluorescence (GIF) and immunohistochemistry. CA-neurons are found both in the central and peripheral nervous system. In the brain, about 50 CA-neurons are present in the perikaryal layer together with numerous CA fibres. Two pairs of CA perikarya are characteristic for each ganglion of the ventral nerve cord. CA-neurites in the ventral nerve cord are mainly arranged in 4 strands paralleling the longitudinal axis of the worm. Fluorescent neurons with receptive ciliary structures are present in body appendages (antennae, palps, urites, parapodial cirri), in the body-wall, and within the oesophageal wall. Furthermore, a subepidermal nerve net of free CA nerve endings has been found. After incubation of specimens with dopamine prior to the development of GIF more fluorescent perikarya could be observed; the fluorescence was also intensified. Pre-incubation with reserpine reduced the intensity of GIF. Results of high pressure liquid chromatography and immunostaining with a polyclonal antibody against a dopamine-glutaraldehyde-complex suggest that dopamine is the major CA transmitter. It is thought that dopaminergic neurons together with ciliary receptive structures act as mechano- and/or chemoreceptors.
Similar content being viewed by others
References
Clark ME (1966) Histochemical localisation of monoamines in the nervous system of the polychaete Nephtys. Proc R Soc Lond (Biol) 165:308–325
Coulon J, Bessone R (1979) Autoradiographic detection of indolamine and catecholamine neurons in the nervous system of Owenia fusiformis (Polychaeta, Annelida). Cell Tissue Res 198:95–104
Dacey DM (1988) Dopamine-accumulating retinal neurons revealed by in vitro fluorescence display a unique morphology. Science 240:1196–1198
Dahl E, Falck B, Mecklenburg C von, Myhrberg H (1963) Adrenergic sensory neurons in invertebrates (abstract). Gen Comp Endocrinol 3:693
Dhainaut-Courtois N (1972) Etude en microscopie électronique et fluorescence des médiateurs chimiques du système nerveux des Nereidae (Annélides Polychètes) Z Zellforsch 126:90–113
Dhainaut-Courtois N, Golding DW (1988) Nervous system. In: Westheide W, Hermans CO (eds) The ultrastructure of the Polychaeta. Microfauna Marina, vol 4. Fischer, Stuttgart, pp 89–110
Dhainaut-Courtois N, Engelhardt R-P, Dhainaut A (1979a) Etude cytophysiologique des systèmes monoaminergiques et cholinergique des Nereis (Annélides Polychètes). I. Systeme nerveux périphérique et jonctions neuromusculaires. Arch Biol (Bruxelles) 90:225–244
Dhainaut-Courtois N, Engelhardt R-P, Dhainaut A (1979b) Etude cytophysiologique des systèmes monoaminergiques et cholinergique des Nereis (Annélides, Polychètes). II. Système nerveux central. Arch Biol (Bruxelles) 90:273–288
Dietzel ID, Gottmann K (1988) Development of dopamine-containing neurons and dopamine uptake in embryos of Hirudo medicinalis. Dev Biol 128:277–283
Dorsett DA (1978) Organization of the nerve cord. In: Mill PJ (ed) Physiology of annelids. Academic Press, London, pp 115–160
Ehinger B, Myhrberg HE (1971) Neuronal localisation of dopamine, noradrenaline, and 5-hydroxytryptamine in the central and peripheral nervous system of Lumbricus terrestris (L.). Histochemistry 28:265–275
Elofsson R, Falck B, Lindvall O, Myhrberg H (1977) Evidence for new catecholamines of related amino acids in some invertebrate sensory neurons. Cell Tissue Res 182:525–536
Falck B, Hillarp NA, Thieme G, Torp A (1962) Fluorescence of catecholamines and related compounds with formaldehyde. J Histochem Cytochem 10:348–354
Geffard M, Buijs RM, Seguela P, Pool CW, Moal M le (1984) First demonstration of highly specific and sensitive antibodies against dopamine. Brain Res 294:161–165
Grothe C, Seidl K, Pfannenstiel HD (1987) Cytochemical and biochemical characterization of neurosecretory material in the brain of an annelid, Ophryotrocha puerilis (Polychaeta). Gen Comp Endocrinol 68:1–5
Haffner K von (1959) Über den Bau und den Zusammenhang der wichtigsten Organe des Kopfendes von Hyalinoecia tubicula MALMGREN (Polychaeta, Eunicidae, Onuphidinae), mit Berücksichtigung der Gattung Eunice, Zool Jb Anat 77:133–192
Lent CM (1982) Fluorescent properties of monoamine neurons following glyoxylic acid treatment of intact leech ganglia. Histochemistry 75:77–89
Lindvall O, Björklund A (1974) The glyoxylic acid fluorescence histochemical method: a detailed account on the methodology for visualisation of central catecholamine neurons. Histochemistry 39:97–127
Marsden JR, Coleman C, Richard R, Jost J, Cain H (1981) Uptake of tritium labelled biogenic amines by the prostomium of the polychaete Nereis virens (Sars) (Annelida). Tissue Cell 13:269–282
Müller T, Unsicker K (1981) High performance liquid chromatography with electrochemical detection as a highly efficient tool for studying catecholaminergic systems. I. Quantification of noradrenaline, adrenaline, and dopamine in cultured adrenal medullary cells. J Neurosci Methods 4:39–53
Myhrberg HE (1967) Monoaminergic mechanisms in the nervous system of Lumbricus terrestris (L.). Z Zellforsch 81:311–343
Myhrberg HE (1971) Ultrastructural localisation of monoamines in the epidermis of Lumbricus terrestris (L.). Z Zellforsch 117:139–154
Nakajima Y (1987) Localisation of catecholaminergic nerves in larval echinoderms. Zool Sci 4:293–299
Pfannenstiel HD (1973) Zur sexuellen Differenzierung von Ophryotrocha puerilis (Polychaeta, Eunicidae). Mar Biol 20:245–258
Pfannenstiel HD (1982) Modified axonemes and ciliary membranes in three polychaete species. Cell Tissue Res 224:181–188
Pfannenstiel HD, Grothe C (1988) Neurosecretory elements. In: Westheide W, Hermans CO (eds) The ultrastructure of the Polychaeta. Microfauna Marina, vol 4. Fischer, Stuttgart, pp 111–120
Pfannenstiel HD, Spiehl D (1987) Dopamine induces sex reversal in females of Ophryotrocha puerilis (Polychaeta). Cell Differ [Suppl] 20:84
Pfannenstiel HD, Schlawny A, Hamann T, Müller M, Rhode B, Spiehl D (1990) Dopamine and male-female differentiation in a hermaphroditic polychaete. In: Epple A, Scanes CG, Stetson MD (eds) Progress in comparative endocrinology. Wiley-Liss, New York, pp 219–225
Reisinger E (1936) Zur Exkretionsphysiologie von Ophryotrocha puerilis Claparède&Metschnikoff. Thalassia 2:2–24
Schlawny A, Hamann T, Müller MA, Pfannenstiel H-D (1991) Cytophysiology of neurosectory axon terminals in the brain of an annelid (Ophryotrocha puerilis, Polychaeta). A re-evaluation. Cell Tissue Res 264:339–345
Sternberger LA (1970) The unlabeled antibody method of immunocytochemistry. J Histochem Cytochem 18:315
White D, Marsden JR (1978) Microspectrofluorimetric measurements on cells containing biogenic amines in the cerebral ganglion of the polychaete Nereis virens (Sars). Biol Bull 155:395–409
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlawny, A., Hamann, T., Müller, M.A. et al. The catecholaminergic system of an annelid (Ophryotrocha puerilis, Polychaeta). Cell Tissue Res 265, 175–184 (1991). https://doi.org/10.1007/BF00318152
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318152