Summary
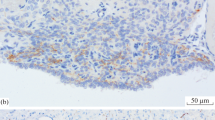
The pineal organ of neonatal rats was transplanted to the frontal part of the cerebral cortex or the cerebral interhemispheric fissure of an isogenic adult rat to determine whether pineal differentiation and pinealopetal innervation are affected by aberrant neuronal influences. Transplants were fixed for immunohistochemistry at 1, 2 and 6 months after transplantation. When treated with an anti-serotonin antibody, cells in transplants from both locations showed intense immunoreactivity and a morphology comparable to intact pinealocytes, indicating that the transplanted pinealocytes had differentiated normally. Tyrosine hydroxylase immunohistochemistry revealed that new catecholamine fibers of central nervous origin extended only into the periphery and not into the core of transplants grafted within the cortex. However, numerous catecholamine fibers were found in transplants placed in the interhemispheric fissure. These fibers were often accompanied by blood vessels, suggesting that they derived from sympathetic ganglia. Serotonin fibers, which are densely distributed in the cerebral cortex, were seldom found to enter transplants from both locations. These observations indicate that pineal cells express their characteristic properties even when transferred to a foreign milieu and that they do not receive novel innervation from the central nerves that normally do not innervate the intact pineal body; the transplant thereby retains the property of selective pinealopetal innervation.
Similar content being viewed by others
References
Aguado LI, Benelbaz GA, Gutierrez LS, Rodríguez EM (1977) Ultrastructure of the rat pineal gland grafted under the kidney capsule. Cell Tissue Res 176:131–142
Araki M, Watanabe K, Tokunaga F, Nonaka T (1988) Phenotypic expression of photoreceptor and endocrine cell properties by cultured pineal cells of the newborn rat. Cell Differ Dev 25:155–164
Bäckström M, Olson L, Seiger Å (1976) N-acetyltransferase and hydroxyindole-O-methyltransferase activity in intraocular pineal transplants: diurnal rhythm as evidence for a functional sympathetic adrenergic innervation. Acta Physiol Scand 96:64–71
Cozzi B, Møller M (1988) Indications for the presence of two populations of serotonin-containing pinealocytes in the pineal complex of the golden hamster (Mesocricetus auratus). An immunohistochemical study. Cell Tissue Res 252:115–122
Gittes RF, Chu EW (1965) Reversal of the effect of pinealectomy in female rats by multiple isogenic pineal transplants. Endocrinology 77:1061–1067
Gupta TKD (1969) Suppression of development of pineal gland by multiple pineal transplants in prepubertal rats. Acta Anat 73:142–147
Hökfelt T, Martensson R, Björklund A, Goldstein M (1984) Distributional map of tyrosine hydroxylase immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 2: classical transmitters in the CNS, Part 1. Elsevier, Amsterdam, pp 277–379
Kappers JA (1960) The development, topographical relations and innervation of the epiphysis cerebri in the albino rat. Z Zellforsch 52:163–215
Klein DC, Auerbach DA, Namboodiri MAA, Wheler GHT (1981) Indole metabolism in the mammalian pineal gland. In: Reiter RJ (ed) The pineal gland, vol 1: anatomy and biochemistry. CRC Press, Boca Raton, Florida, pp 199–227
Lingappa JR, Zigmond RE (1987) Pineal transplants in oculo: limitations of the ability of collateral sprouts of foreign neurons to establish normal function. Neuroscience 7:3525–3528
Matsuura T, Takeuchi Y, Sano Y (1983) Immunohistochemical and electron microscopical studies on serotonin-containing nerve fibers in the pineal organ of the rat. Biomed Res 4:261–270
Møller M (1981) The ultrastructure of the deep pineal gland of the Mongolian gerbil and mouse: granular vesicle localization and innervation. In: Matthews CD, Seamark RF (eds) Pineal function. Elsevier North-Holland, Amsterdam, pp 257–266
Moore RY (1975) Pineal transplants to the anterior chamber of the eye: evidence for functional reinnervation. Exp Neurol 49:617–621
Nagatsu I (1983) Immunohistochemistry of biogenic amines and immunoenzyme-histochemistry of catecholamine-synthesizing enzymes. In: Parvez S, Nagatsu T, Nagatsu I, Parvez H (eds) Methods in biogenic amino research. Elsevier, Amsterdam, pp 873–909
Oksche A, Hartwig HG (1979) Pineal sense organs—components of photoendocrine systems. Prog Brain Res 52:113–130
Olson L, Malmfors T (1970) Growth characteristics of adrenergic nerves in the adult rat. Acta Phisiol Scand 348:9–144
Osman P, Welschen RW, Moll J (1972) Anti-gonadotropic and anti-growth effects of the pineal gland in immature female rats. Neuroendocrinol 10:121–128
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, New York
Reiter RJ (1981) The mammalian pineal gland: structure and function. Am J Anat 162:287–313
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-anti horseradish peroxidase) and its use in identification of spirochates. J Histochem Cytochem 18:315–333
Takeuchi Y, Kimura H, Sano Y (1982) Immunohistochemical demonstration of the distribution of serotonin neurons in the brain stem of the rat and cat. Cell Tissue Res 224:247–267
Vigh B, Vigh-Teichmann I (1981) Light- and electron-microscopic demonstration of immunoreactive opsin in the pinealocytes of various vertebrates. Cell Tissue Res 221:451–463
Vries RAC De (1972) Abolition of the effect of pinealectomy on hypothalamic magnocellular neurosecretory activity in male rats by hypothalamic pineal implants. Neuroendocrinology 9:358–364
Wakabayashi H, Shimada K, Aizawa Y (1985) Determination of serotonin and melatonin in rat pineal gland by highperformance liquid chromatography with ultraviolet and fluorometric dual detection. Chem Pharmacol Bull 33:3875–3880
Walker RF, Aloyo V (1985) Norepinephrine stimulates serotonin secretion from rat pineal glands in vitro. Brain Res 343:188–189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nonaka, T., Araki, M., Kimura, H. et al. Transplantation of the rat pineal organ to the brain: pinealocyte differentiation and innervation. Cell Tissue Res 260, 273–278 (1990). https://doi.org/10.1007/BF00318630
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318630