Summary
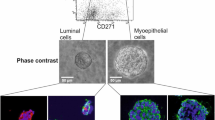
Fragments of human breast epithelium, devoid of all stromal and basal lamina components, which maintain their in vivo topological organisation can be cultured for up to 28 days within a reconstituted rat-tail-derived collagen matrix. These organoids initially undergo a loss of structural and 3-dimensional organisation, typified by loss of lumina formed by epithelial cells, and myosin from myoepithelial cells. Their subsequent reorganisation is dependent on the presence of serum, insulin, hydrocortisone, and cholera toxin in tissue culture medium. After this preliminary phase, a reduction in the concentration of serum, insulin, hydrocortisone, and cholera toxin is necessary to allow the structural differentiation of epithelial and myoepithelial cells. The myoepithelial cells also regain their ability to produce the basal lamina component laminin. The use of bovine-dermal collagen as the matrix, rather than rat-tail-derived collagen is shown to result in more stable organisation and differentiation of the organoids. The successful use of single-cell pellets (derived by trypsinisation of the organoids) in place of organoids in such cultures illustrates that there is no requirement for pre-existing cell/ cell contact or topological organisation of cells prior to embedding within the collagen matrix.
Similar content being viewed by others
References
Bornstein MB (1958) Reconstituted rat-tail collagen used as a substrate for tissue culture on coverslips in Maximow slides and roller tubes. Lab Invest 7:134–137
Easty GC, Easty DM, Monaghan P, Ormerod MG, Neville AM (1980) Preparation and identification of human breast epithelial cells in culture. Int J Cancer 26:577–584
Ehrmann RL, Gey GO (1956) The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst 16:1375–1390
Foster CS, Smith CA, Dinsdale EA, Monaghan P, Neville AM (1983) Human mammary gland morphogenesis in vitro: The growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies, and autoradiography. Dev Biol 96:197–216
Goldman RD, Knipe DM (1972) The functions of cytoplasmic fibres in non-muscle cell motility. Cold Spring Harbor Symp Quant Biol 37:523–534
Goldman RD, Berg G, Bushnell A, Chang CM, Dickerman L, Hopkins N, Miller ML, Pollack R, Wang E (1973) Fibrillar systems in cell motility. Locomotion of tissue cells. Ciba Foundation Symp 14:83–107
Gusterson BA, Williams J, Bunnage H, O'Hare MJ, Dubois J-D (1984) Human breast epithelium transplanted into nude mice. Proliferation and milk protein production in response to pregnancy. Virchows Arch [A] 404:325–333
Lasfargues EY (1957) Cultivation and behaviour in vitro of the normal mammary epithelium of the adult mouse. Anat Rec 127:117–129
Michalopoulos G, Pilot HC (1975) Primary culture of parenchymal liver cells on collagen membranes. Exp Cell Res 94:70–78
Mollenhauer HH (1964) Plastic embedding mixtures for use in electron microscopy. Stain Technol 39:111–114
Puchtler H, Leblond CP (1958) Histochemical analysis of cell membranes and associated structures as seen in the intestinal epithelium. Am J Anat 102:1–31
Warburton MJ, Mitchell D, Ormerod EJ, Rudland PS (1982) Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating and invulating rat mammary gland. J Histochem Cytochem 30:667–676
Yang J, Richards J, Bowman P, Guzman R, Enami J, McCormick K, Hamamoto S, Pitelka D, Nandi S (1979) Sustained growth and three-dimensional organisation of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci USA 76:3401–3405
Yang J, Guzman R, Richards J, Jentoft V, DeVault MR, Wellings SR, Nandi S (1980) Primary culture of human mammary epithelial cells embedded in collagen gels. J Natl Cancer Inst 65:337–343
Yang J, Elias JJ, Petrakis NL, Wellings SR, Nandi S (1981) Effects of hormones and growth factors on human mammary epithelial cells in collagen gel culture. Cancer Res 41:1021–1027
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, C.A., Bunnage, H.J., Monaghan, P. et al. Human breast epithelium in vitro: The re-expression of structural and functional cellular differentiation in long-term culture. Cell Tissue Res. 247, 433–440 (1987). https://doi.org/10.1007/BF00218324
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218324