Abstract
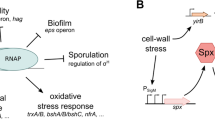
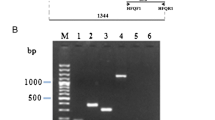
The expression of thegsiB gene ofBacillus subtilis in response to a wide variety of stress conditions was analysed, and the results provide evidence thatgsiB is subject to a σB regulation. Primer extension experiments established identical start points forgsiB transcription during growth and after the induction by heat shock, salt or ethanol stress, and glucose limitation. The sequence upstream of the transcription start point shows great similarity to the sequences of σB promoters ofB. subtilis. σB was absolutely required for the increase ingsiB mRNA level and in the synthesis rate of GsiB in response to various stimuli. Measurements of the ATP pool indicated that changes in the level of ATP might be one of the signals that regulate the activity of σB inB. subtilis.
Similar content being viewed by others
References
Alper S, Duncan L, Losick R (1994) An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor inB. subtilis. Cell 77:1–20
Belitsky BR, Shakylov RS (1980) Amount of guanosine polyphosphate and the level of stable RNA synthesis inB. subtilis cells upon inhibition of protein synthesis. Molekularnaja Biologija 14:1343–1353
Benson AK, Haldenwang WG (1992) Characterization of a regulatory network that controls σB expression inB. subtilis. J Bacteriol 174:749–757
Benson AK, Haldenwang WG (1993a) The σB promoter of theBacillus subtilis sigB operon is induced by heat shock. J Bacteriol 175:1929–1935
Benson AK, Haldenwang WG (1993b)Bacillus subtilis σB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA 90:2330–2334
Boylan SA, Thomas SM, Price CW (1991) Genetic method to identify regulons controlled by nonessential elements: isolation of a gene dependent on alternate transcription factor σB ofBacillus subtilis. J Bacteriol 173:7856–7866
Boylan SA, Rutherford A, Thomas SM, Price CW (1992) Activation ofBacillus subtilis transcription factor σB by a regulatory pathway responsive to stationary-phase signals. J Bacteriol 174:3695–3706
Boylan SA, Redfield AR, Price CW (1993a) Transcription factor σB ofBacillus subtilis controls a large stationary-phase regulon. J Bacteriol 175:3957–3963
Boylan SA, Redfield AR, Brody MS, Price CW (1993b) Stress-induced activation of the σB transcription factor ofBacillus subtilis. J Bacteriol 175:7931–7937
Dufour A, Haldenwang WG (1994) Interactions between aBacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV). J Bacteriol 176:1813–1820
Duncan L, Losick R (1993) SpoIIAB is an anti-sigma factor that binds to and inhibits transcription by regulatory protein σF fromBacillus subtilis. Proc Natl Acad Sci USA 90:2325–2329
Hecker M, Völker U (1990) General stress proteins inBacillus subtilis. FEMS Microbiol Ecol 74:197–214
Igo M, Losick R (1986) Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme inBacillus subtilis. J Mol Biol 191:615–624
Igo M, Lampe M, Ray C, Schafer W, Moran CP, Losick R (1987) Genetic studies of a secondary RNA polymerase sigma factor inBacillus subtilis. J Bacteriol 169:3464–3469
Igo M, Lampe M, Losick R (1988) Structure and regulation of aBacillus subtilis gene that is transcribed by E σB form of RNA polymerase holoenzyme. In: Ganesan AT, Hoch JA (eds) Genetics and biotechnology of Bacilli, vol. 2. Academic Press New York, pp 151–156
Kalman S, Duncan ML, Thomas SM, Price CW (1990) Similar organization of thesigB andspoIIA operons encoding alternate sigma factors ofBacillus subtilis RNA polymerase. J Bacteriol 172:5575–5585
Kirchman PA, Degrazia H, Kellner EM, Moran CP (1993) Forespore-specific disappearance of the sigma-factor antagonist SpollAB — Implications for its role in determination of cell fate inBacillus subtilis. Mol Microbiol 8:663–671
Lundin A, Thore A (1975) Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol 30:713–721
Majumdar D, Avissar YJ, Wyche JH (1991) Simultaneous and rapid isolation of bacterial and eucaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques 11:94–101
Min KT, Hilditch CM, Diederich B, Errington J, Yudkin MD (1993) σF, the first compartment-specific transcription factor ofBacillus subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell 74:735–742
Moran CP, Lang N, Losick R (1981) Nucleotide sequence of aBacillus subtilis promoter recognized byBacillus subtilis RNA polymerase containing σ37. Nuclic Acids Res 9:5979–5990
Mueller JP, Mathiopoulos C, Slack FJ, Sonenshein AL (1991) Identification ofBacillus subtilis adaptive response genes by subtractive differential hybridization. Res Microbiol 142: 805–813
Mueller JP, Bukusoglu G, Sonenshein AL (1992) Transcriptional regulation ofBacillus subtilis glucose starvation-inducible genes — Control ofgsiA by the ComP-ComA signal transduction system. J Bacteriol 174:4361–4373
Ollington JF, Haldenwang WG, Huynh TV, Losick R (1981) Developmentally regulated transcription in a cloned segment of theBacillus subtilis chromosome. J Bacteriol 147:432–442
Richter A, Hecker M (1986) Heat shock proteins inBacillus subtilis. A two-dimensional electrophoresis study. FEMS Microbiol Lett 36:69–71
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Smith I, Paress P, Cabane K, Dubnau E (1980) Genetics and physiology of therel system ofBacillus subtilis. Mol Gen Genet 178:271–279
Stülke J, Hanschke R, Hecker M (1993) Temporal activation of glucanase synthesis inBacillus subtilis is mediated by the GTP pool. J Gen Microbiol 39:2041–2045
Varon D, Boylan SA, Okamoto K, Price CW (1993)Bacillus subtilis gtaB encodes UDP-Glucose-pyrophosphorylase and is controlled by stationary-phase transcription factor σB. J Bacteriol 175:3964–3971
Völker U, Mach H, Schmid R, Hecker M (1992) Stress proteins and cross protection by heat shock and salt stress inBacillus subtilis. J Gen Microbiol 138:2125–2135
Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M (1994) Analysis of the induction of general stress proteins ofBacillus subtilis. Microbiology 140:741–752
Völker U, Dufour A, Haldenwang WG (1995) TheBacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J Bacteriol 177:114–122
Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W (1992) Cloning, sequencing, and molecular analysis of thednaK locus fromBacillus subtilis. J Bacteriol 174:3300–3310
Wise AA, Price C (1995) Four additional genes in thesigB operon ofBacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J Bacteriol 177:123–133
Author information
Authors and Affiliations
Additional information
Communicated by J. F. Lengeler
Rights and permissions
About this article
Cite this article
Maul, B., Völker, U., Riethdorf, S. et al. σB-dependent regulation ofgsiB in response to multiple stimuli inBacillus subtilis . Molec. Gen. Genet. 248, 114–120 (1995). https://doi.org/10.1007/BF02456620
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02456620