Summary
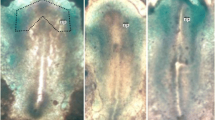
Scanning electron microscopic studies revealed that Concanavalin A (ConA) induces characteristic changes of the cell surface and the cell architecture of the presumptive ectoderm associated with differentiation into neural tissues. In Con A-treated cells, the filopodia with which cells were connected to each other disappeared from the interior (blastocoelic) surface and the cellular adhesivity decreased significantly. Thereafter, the cells underwent from those of the control explants. After cultivation for 60 h, a certain pattern of cell arrangement, which resembled the architecture of neural tissues, was observed among randomly arranged cells in the explants treated with Con A. The morphological changes specifically observed in Con A-treated explants were different from those found in explants treated with succinyl Con A (S-Con A) orDolichos biflorus agglutinin (DBA), which is unable to induce formation of the neural tissues. The molecular organization of the plasma membrane appears to be important in the mechanism of neural induction.
Similar content being viewed by others
References
Ash JF, Singer SJ (1976) Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosincontaining filaments. Proc Natl Acad Sci USA 73:4575–4579
Carbonetto S, Argon Y (1980) Lectins induce the redistribution and internalization of receptors on the surface of cultured neurons. Dev Biol 80:364–378
Carter WG, Hakomori S (1977) Isolation and partial characterization of “galactoprotein a” (LETs) and “galactoprotein b” from Hamster embryo fibroblasts. Biochem Biophys Res Commun 76:299–308
Carter WG, Fukuda M, Lingwood C, Hakomori S (1978) Chemical composition, gross structure, and organization of transformationsensitive glycoproteins. Ann N Y Acad Sci 312:160–177
Edelson RJ, Cohn ZA (1974a) Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phagolysosome formation. J Exp Med 140:1364–1386
Edelson RJ, Cohn ZA (1974b) Effects of concanavalin A on mouse peritoneal macrophages. II. Metabolism of endocytized proteins and reversibility of effects by mannose. J Exp Med 140:1387–1403
Grunz H, Staubach J (1979) Changes of the cell surface change of amphibian ectoderm after induction. Wilhelm Roux's Arch 186:77–80
Grunz H, Multier-Lajous A-M, Herbst P, Arkenberg G (1975) The differentiation of isolated amphibian ectoderm with or without treatment with a inductor. A scanning electron microscope study. Wilhelm Roux's Arch 178:277–284
Holtfreter J (1943) Properties and functions of the surface coat in amphibian embryos. J Exp Zool 93:251–323
Keller RE (1980) The cellular basis of epiboly: An SEM study of deep-cell rearrangement during gastrulation inXenopus laevis J Embryol Exp Morphol 60:201–234
Keller RE, Schoenwolf GC (1977) An SEM study of cellular morphology, contact, and arrangement, as related to gastrulation inXenopus laevis. Wilhelm Roux's Arch 182:165–186
Kohonen J, Paranko J (1978) Surface topography of isolated gastrula ectoderm ofTriturus vulgaris. Med Biol 56:328–332
Kojima K, Sato C (1979) Further evidence for concanavalin A-induced translocation of chondroitin sulfate at the cell surface of mammary tumor cells. Cell Struc Func 4:227–233
Letourneau PC (1976) Inhibition of intercellular adhesion by concanavalin A is associated with concanavalin A-mediated redistribution of surface receptors. J Cell Biol 80:128–140
Monroy A, Baccetti B, Denis-Donini A (1976) Morphological changes of the surface of the egg ofXenopus laevis in the course of development. III. Scanning electron microscopy of gastrulation. Dev Biol 49:250–259
Moran D, Mouradian WE (1975) A scanning electron microscopic study of the appearance and localization of cell surface material during amphibian gastrulation. Dev Biol 46:422–429
Nakatsuji, N (1976) Studies on the gastrulation of amphibian embryos: Ultrastructure of the migrating cells of anurans. Wilhelm Roux's Arch 180:229–240
Nicolson BL (1974) The interactions of lectins with animal cell surface. Inter Rev Cytl 39:89–190
Okada YK, Ichikawa M (1947) Normal table of Triturus pyrrhogaster. Appl J Exp Morphol 3:1–6
Perry MM (1975) Microfilaments in the external surface layer of the early amphibian embryo. J Embryol Exp Morphol 33:127–146
Petty HR, Ware BR (1979) Macrophage response to concanavalin A: Effect of surface crosslinking on the electrophoretic mobility distribution. Proc Natl Acad Sci USA 76:2278–2282
Prinz R, Von Figura K (1978) Adhesion of human skin fibrobiasts Effect of tetravalent, divalent and monovalent concanavalin A. Exp Cell Res 112:275–279
Rauvala HR, Carter WG, Hakomori S (1981) Studies on cell adhesion and recognition. I. Extent and specificity of cell adhesion triggered by carbohydrate-reactive proteins (glycosidases and lectins) and by fibronectin. J Cell Biol 88:127–137
Sato C, Miyazawa T, Nishizawa K, Kojima K, Okayama M (1979) Changes in the organization and biosynthesis of cell surface acidie sugars during the phytohem-agglutinin-induced blasto formation of human T-lymphocytes. Exp Cell Res 124:285–292
Schlessinger J, Koppel DE, Axelrod D, Jacobson K, Webb WW, Elson EL (1976) Lateral transport on cell membranes: Mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci USA 73:2409–2413
Takata K, Yamamoto KY, Ozawa R (1981) Use of lectins as probes for analyzing embryonic induction. Wilhelm Roux's Arch 190:92–96
Tarin D (1971) Scanning electron microscopical studies of the embryonic surface during gastrulation and neurulation inXenopus laevis. J Anat 109:535–547
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, K.Y., Ozawa, R., Takata, K. et al. Cell surface changes of the presumptive ectoderm following neural-inducing treatment by concanavalin A. Wilhelm Roux' Archiv 190, 313–319 (1981). https://doi.org/10.1007/BF00863268
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00863268