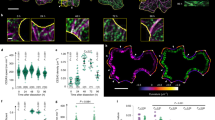
Abstract
The involvement of calmodulin (CaM) in wound-induced cytoplasmic contractions in E. verticillata was investigated. Indirect immunofluorescence of CaM in intact cells showed a faint, reticulate pattern of fluorescence in the cortical cytoplasm. Diffuse fluorescence was evident deeper within the cytoplasm. In contracted cells, CaM co-localizes with actin in the cortical cytoplasm in extensive, longitudinal bundles of microfilaments (MFs), and in an actin-containing reticulum. No association of CaM with tubulin was ever observed in the cortical cytoplasm at any stage of wound-healing. When contraction rates in wounded cells are measured, a lag period of 2 min is followed by a rapid, steady rate of movement over the subsequent 10 min. The delay in the initiation of longitudinal contraction corresponds to the time necessary for the assembly of the longitudinal MF bundles. Cytoplasmic motility was inhibited in a dose-dependent manner by CaM antagonists. In these inhibited cells, MF bundles did not assemble, or were poorly formed. In the latter case, CaM was always found associated with MFs. These results indicate a direct spatial and temporal correlation between CaM and actin, and a potential role for CaM in regulating the formation of functional MF bundles during wound-induced cytoplasmic contraction in Ernodesmis.
Similar content being viewed by others
Abbreviations
- CaM:
-
calmodulin
- DMSO:
-
dimethyl sulfoxide
- EGTA:
-
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- MF(s):
-
microfilament(s)
- MT(s):
-
microtubule(s)
- TFP:
-
trifluoperazine
- w-5:
-
N-(6-aminohexyl)-1-naphthalenesulfonamide
- W-7:
-
N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide
References
Allan, E., Hepler, P.K. (1989) Calmodulin and calcium-binding proteins. In: The biochemistry of plants: a comprehensive treatise, vol. 15, pp. 455–484, Marcus, A., ed. Academic Press, San Diego
Biro, R.L., Daye, S., Serlin, B.S., Terry, M.E., Datta, N., Sopory, S.K., Roux, S.J. (1984) Characterization of oat calmodulin and radioimmunoassay of its subcellular distribution. Plant Physiol. 75, 382–386
Burgess, W.H., Jemiolo, D.K., Kretsinger, R.H. (1980) Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim. Biophys. Acta 623, 257–270
Burgess, W.H., Schleicher, M., Van Eldik, L.J., Watterson, D.M. (1983) Comparative studies of calmodulin. In: Calcium and cell function, vol. IV, pp. 209–261, Cheung, W.Y., ed. Academic Press, New York
Cotton, G., Vanden Driessche, T. (1987) Identification of calmodulin in Acetabularia: its distribution and physiological significance. J. Cell Sci. 87, 337–347
Dauwalder, M., Roux, S.J., Hardison, L. (1986) Distribution of calmodulin in pea seedlings: immunocytochemical localization in plumules and root apices. Planta 168, 461–470
Dedman, J.R., Welsh, M.J., Means, A.R. (1978) Ca2+-dependent regulator: production and characterization of a monospecific antibody. J. Biol. Chem. 253, 7515–7521
Gilroy, S., Blowers, D.P., Trewavas, A.J. (1987) Calcium: a regulation system emerges in plant cells. Development 100, 181–184
Glenney, J.R., Jr., Weber, K. (1980) Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J. Biol. Chem. 255, 10551–10554
Grolig, F., Williamson, R.E., Parke, J., Miller, C., Anderton, B.H. (1988) Myosin and Ca2+-sensitive streaming in the alga Chara: detection of two polypeptides reacting with a monoclonal antimyosin and their localization in the streaming endoplasm. Eur. J. Cell Biol. 47, 22–31
Hidaka, H., Asano, M., Tanaka, T. (1981) Activity-structure relationship of calmodulin antagonists: naphthalenesulfonamide derivatives. Mol. Pharmacol. 20, 571–578
Kikuyama, M., Tazawa, M. (1982) Ca2+ ion reversibly inhibits the cytoplasmic streaming of Nitella. Protoplasma 113, 241–243
Kohno, T., Shimmen, T. (1987) Ca2+-induced fragmentation of actin filaments in pollen tubes. Protoplasma 141, 177–179
Kohno, T., Shimmen, T. (1988) Mechanism of Ca2+ inhibition of cytoplasmic streaming in lily pollen tubes. J. Cell Sci. 91, 501–509
Kyhse-Anderson, J. (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Meth. 10, 203–209
La Claire, J.W., II (1982) Cytomorphological aspects of wound healing in selected Siphonocladales (Chlorophyceae). J. Phycol. 18, 379–384
La Claire, J.W., II (1984) Cell motility during wound healing in giant algal cells: contraction in detergent-permeabilized cell models of Ernodesmis. Eur. J. Cell Biol. 33, 180–189
La Claire, J.W., II (1987) Microtubule cytoskeleton in intact and wounded coenocytic green algae. Planta 171, 30–42
La Claire, J.W., II (1989) Actin cytoskeleton in intact and wounded coenocytic green algae. Planta 177, 47–57
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Lessard, J.L. (1988) Two monoclonal antibodies to actin: One muscle selective and one generally reactive. Cell Motil. Cytoskel. 10, 349–362
Lonergan, T.A. (1985) Regulation of cell shape in Euglena gracilis. IV. Localization of actin, myosin, and calmodulin. J. Cell Sci. 77, 197–208
Margolis, R.L. (1983) Calcium and microtubules. In: Calcium and cell function, vol. IV, pp. 313–335, Cheung, W.Y., ed. Academic Press, New York
Marshak, D.R., Watterson, D.M., Van Eldik, L.J. (1981) Calciumdependent interaction of S100b, troponin C, and calmodulin with an immobilized phenothiazine. Proc. Natl. Acad. Sci. USA 78, 6793–6797
Means, A.R., George, S.E. (1988) Calmodulin regulation of smooth-muscle myosin lightchain kinase. J. Cardiovasc. Pharmacol. 12 (Suppl. 5), S25-S29
Menzel, D., Elsner-Menzel, C. (1989) Induction of actin-based cytoplasmic contraction in the siphonous green alga Acetabularia (Chlorophyceae) by locally restricted calcium influx. Bot. Acta 102, 164–171
Miernyk, J.A., Fang, T.K., Randall, D.D. (1987) Calmodulin antagonists inhibit the mitochondrial pyruvate dehydrogenase complex. J. Biol. Chem. 262, 15338–15340
Mische, S.M., Mooseker, M.S., Morrow, J.S. (1987) Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J. Cell. Biol. 105, 2837–2845
Piazza, G.J. (1988) Calmodulin in plants. In: Calcium binding proteins, vol. I, pp. 127–143, Thompson, M.P., ed. CRC Press, Boca Raton, Fla., USA
Piazza, G.A., Wallace, R.W. (1985) Calmodulin accelerates the rate of polymerization of human platelet actin and alters the structural characteristics of actin filaments. Proc. Natl. Acad. Sci. USA 82, 1683–1687
Pritchard, K., Moody, C.J. (1986) Caldesmon: a calmodulin-binding actin-regulatory protein. Cell Calcium 7, 309–327
Qiao, L., Grolig, F., Jablonsky, P.P., Williamson, R.E. (1989) Myosin heavy chains: detection by immunoblotting in higher plants and localization by immunofluorescence in the alga Chara. Cell Biol. Int. Rep. 13, 107–118
Roberts, D.M., Lukas, T.J., Watterson, D.M. (1986) Structure, function, and mechanism of action of calmodulin. CRC Crit. Rev. Plant Sci. 4, 311–339
Sachs, L. (1984) Applied statistics: A handbook of techniques, 2nd edn., transl, by Z. Reynarowych. Springer, New York Berlin Heidelberg
SAS Institute Inc. (1985) SAS User's Guide: Statistics Version 5 Edition. SAS Institute Inc., Cary, N.C., USA
Schnepf, E., Volkmann, K. (1974) Inhibition of traumatotactic movement of nuclei in Tradescantia leaf epidermis. II. Effects of heavy water, ethionine, Ca2+, and cyclic AMP. Protoplasma 81, 313–321
Scott, J.A., Fischman, A.J., Khaw, B.-A., Rabito, C.A. (1988) Phenothiazine-mediated depolarization of the plasma membrane in a renal cell line. Biochem. Pharmacol. 37, 3785–3787
Serlin, B.S., Roux, S.J. (1984) Modulation of chloroplast movement in the green alga Mougeotia by the Ca2+ ionophore A23187 and by calmodulin antagonists. Proc. Natl. Acad. Sci. USA 81, 6368–6372
Sharpies, D. (1981) Photodecomposition of some substituted phenothiazines. J. Pharm. Pharmacol. 33, 242–243
Staiger, C.J., Schliwa, M. (1987) Actin localization and function in higher plants. Protoplasma 141, 1–12
Toriyama, H., Jaffe, M.J. (1972) Migration of calcium and its role in the regulation of seismonasty in the motor cell of Mimosa pudica L. Plant Physiol. 49, 72–81
Towbin, H., Staehelin, T., Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Van Eldik, L.J., Wolchok, S.R. (1984) Conditions for reproducible detection of calmodulin and S100B in immunoblots. Biochem. Biophys. Res. Commun. 124, 752–759
Vantard, M., Lambert, A.-M., De Mey, J., Picquot, P., Van Eldik, L.J. (1985) Characterization and immunocytochemical distribution of calmodulin in higher plant endosperm cells: localization in the mitotic apparatus. J. Cell Biol. 101, 488–499
Veigl, M.L., Vanaman, T.C., Sedwick, W.D. (1984) Calcium and calmodulin in cell growth and transformation. Biochim. Biophys. Acta 738, 21–48
Wagner, G., Klein, K. (1978) Differential effect of calcium on chloroplast movement in Mougeotia. Photochem. Photobiol. 27, 137–140
Wagner, G., Haupt, W., Laux, A. (1972) Reversible inhibition of chloroplast movement by cytochalasin B in the green alga Mougeotia. Science 176, 808–809
Wagner, G., Valentin, P., Dieter, P., Marme, D. (1984) Identification of calmodulin in the green alga Mougeotia and its possible function in chloroplast reorientational movement. Planta 162, 62–67
Wang, K., Feramisco, J.R., Ash, J.F. (1982) Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 85, 514–562
Wick, S.M. (1988) Immunolocalization of tubulin and calmodulin in meristematic plant cells. In: Calcium binding proteins, vol. II, pp. 21–45, Thompson, M.P., ed. CRC Press, Boca Raton, Fla., USA
Author information
Authors and Affiliations
Additional information
We are especially grateful to: Dr. J.A. West (University of California, Berkeley) for the original algal isolates; Dr. L. Van Eldik (Vanderbilt University School of Medicine) and Dr. J.L. Lessard (University of Cincinnati College of Medicine) for graciously providing CaM and actin antibodies, respectively; Dr. S.J. Roux (University of Texas, Austin) for the gift of purified oat CaM; Dr.H. Green (Smith, Kline and French Laboratories, Philadelphia, Penn., USA) for providing the trifluoperazine; and M.E.T. Scioli for assistance with the statistical analyses. Portions of this work were supported by National Science Foundation grant DCB 8402345 and U.S. Department of Agriculture grant 87-CRCR-1-2545 to J.W.L.
Rights and permissions
About this article
Cite this article
Goddard, R.H., La Claire, J.W. Calmodulin and wound healing in the coenocytic green alga Ernodesmis verticillata (Kützing) Børgesen. Planta 183, 281–293 (1991). https://doi.org/10.1007/BF00197800
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197800