Abstract
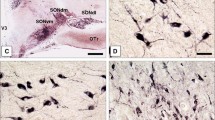
The mRNA levels of secretogranin II (SgII), VGF and peptidylglycine alpha-amidating monooxygenase (PAM) were studied in brains of salt loaded rats by in situ hybridization. In these rats the levels of the message for secretogranin II and VGF were increased in the paraventricular, supraoptic and retrochiasmatic nuclei and in the subfornical organ. The increases ranged from 416 to 721% for SgII and from 778 to 890% for VGF. The PAM message was also elevated in these brain regions; however, the maximal increase was only 221%. We conclude that the message for all secretory peptides investigated so far, i.e. vasopressin, galanin, secretogranin II and VGF are upregulated to a similar degree in the hypothalamus of salt-located rats. The relative increase in mRNA for the enzyme peptidylglycine alpha-amidating monooxygenase occurred to a much lower extent, and was comparable to the limited changes previously seen for carboxypeptidase H.
Similar content being viewed by others
References
Bahner U, Geiger H, Palkovits M, Ganten D, Michel J, Heidland A (1990) Atrial natriuretic peptides in brain nuclei of rats with inherited diabetes insipidus (Brattleboro rats). Neuroendocrinology 51:721–727
Braas KM, Stoffers DA, Eipper BA, May V (1989) Tissue specific expression of rat peptidylglycine alpha-amidating monooxygenase activity and mRNA. Mol Endocrinol 3:1387–1398
Cozzi MG, Rosa P, Greco A, Hille A, Huttner WB, Zanini A, De Camilli P (1989) Immunohistochemical localization of secretogranin II in the rat cerebellum. Neuroscience 28:423–441
Eiden LE, Huttner WB, Mallet J, O'Connor DT, Winkler H, Zanini A (1987) A nomenclature proposal for the chromogranin/secretogranin proteins. Neuroscience 21:1019–1021
Eipper BA, Stoffers DA, Mains RE (1992) The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci 15:57–85
Erickson JD, Lloyd R, Trojanowski JQ, Iacangelo A, Eiden E (1992) Sites of synthesis of chromogranins A and B in the human brain. Neuropeptides 21:239–244
Ferri G-L, Levi A, Possenti R (1992) A novel neuroendocrine gene product: selective VGF8a gene expression and immunolocalisation of the VGF protein in endocrine and neuronal populations. Mol Brain Res 13:139–143
Fischer-Colbrie R, Iacangelo A, Eiden LE (1988) Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci USA 85:3240–3244
Fischer-Colbrie R, Gutierrez J, Hsu C-M, Iacangelo A, Eiden LE (1990) Sequence analysis, tissue distribution and regulation by cell depolarization and second-messengers of bovine secretogranin II (chromogranin C) mRNA. J Biol Chem 265:9208–9213
Forss-Petter S, Danielson P, Battenberg E, Bloom F, Sutcliffe JG (1989) Nucleotide sequence and cellular distribution of rat chromogranin B (secretogranin I) mRNA in the neuroendocrine system. J Mol Neurosci 1:63–75
Galindo E, Bader M-F, Aunis D (1991) Regulation of chromogranin A and chromogranin B (secretogranin I) synthesis in bovine cultured chromaffin cells. J Neuroendocrinol 3:669–677
Gerdes HH, Phillips E, Huttner WB (1988) The primary structure of rat secretogranin II deduced from a cDNA sequence. Nucleic Acids Res 16:11811
Grino M, Guillaume V, Boudouresque F, Conte-Devolx B, Maltese J-Y, Oliver C (1990) Glucocorticoids regulate peptidylglycine alpha-amidating monooxygenase gene expression in the rat hypothalamic paraventricular nucleus. Mol Endocrinol 4:1613–1619
Höfle G, Weiler R, Fischer-Colbrie R, Humpel C, Laslop A, Wohlfarter T, Hogue-Angeletti R, Saria A, Fleming PJ, Winkler H (1991) Stimulation of rat adrenal medulla can induce differential changes in the peptide and mRNA levels of chromogranins, neuropeptides and other constituents of chromaffin granules. Regul Pept 32:321–331
Laslop A, Wohlfarter T, Fischer-Colbrie R, Steiner H-J, Humpel C, Saria A, Schmid KW, Sperk G, Winkler H (1989) Insulin hypoglycemia increases the levels of neuropeptide Y and calcitonin gene related peptide, but not of chromogranins A and B, in rat chromaffin granules. Regul Pept 26:191–202
Lassmann H, Hagn C, Fischer-Colbrie R, Winkler H (1986) Presence of chromogranin A, B and C in bovine endocrine and nervous tissues: a comparative immunohistochemical study. Histochem J 18:380–386
Levi A, Eldridge JD, Paterson BM (1985) Molecular cloning of a gene sequence regulated by nerve growth factor. Science 229:393–395
Lightman SL, Young W III (1987) Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol 394:23–39
Lloyd RV, Jin L, Fields K (1990) Detection of chromogranins A and B in endocrine tissues with radioactive and biotinylated oligonucleotide probes. Am J Surg Pathol 14:35–43
Mahata SK, Mahata M, Marksteiner J, Sperk G, Fischer-Colbrie R, Winkler H (1991) Distribution of mRNAs for chromogranins A and B and secretogranin II in rat brain. Eur J Neurosci 3:895–904
Mahata SK, Mahata M, Steiner H-J, Fischer-Colbrie R, Winkler H (1992) In situ hybridization: mRNA levels of secretogranin II, neuropeptides and carboxypeptidase H in brains of saltloaded and Brattleboro rats. Neuroscience 48:669–680
Meister B, Cortés R, Villar MJ, Schalling M, Hökfelt T (1990) Peptides and transmitter enzymes in hypothalamic magnocellular neurons after administration of hyperosmotic stimuli: comparison between messenger RNA and peptide/protein levels. Cell Tissue Res 260:279–297
Miselis RR (1981) The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230:1–23
Ouafik L'H, May V, Saffen DW, Eipper BA (1990) Thyroid hormone regulation of peptidylglycine alpha-amidating monooxygenase expression in anterior pituitary gland. Mol Endocrinol 4:1497–1505
Possenti R, Eldridge JD, Paterson BM, Grasso A, Levi A (1989) A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. EMBO J 8:2217–2223
Rindi G, Buffa R, Sessa F, Tortora O, Solcia E (1986) Chromogranin A, B and C immunoreactivities of mammalian endocrine cells. Distribution, distinction from costored hormones/prohormones and relationship with the argyrophil component of secretory granules. Histochemistry 85:19–28
Rhodes CH, Xu R-Y, Angeletti RH (1990) Peptidylglycine alpha-amidating monooxygenase (PAM) in Schwann cells and glia as well as neurons. J Histochem Cytochem 38:1301–1311
Rosa P, Zanini A (1981) Characterization of adenohypophysial polypeptides by two-dimensional gel electrophoresis. II. Sulfated and glycolysated polypeptides. Mol Cell Endocrinol 24:181–193
Rosa P, Hille A, Lee RWH, Zanini A, De Camilli P, Huttner WB (1985) Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol 101:1999–2011
Salton SRJ (1991) Nucleotide sequence and regulatory studies of VGF, a nervous system-specific mRNA that is rapidly and relatively selectively induced by nerve growth factor. J Neurochem 57:991–996
Salton SRJ, Fischberg DJ, Dong K-W (1991) Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol Cell Biol 11:2335–2349
Schafer MK-H, Stoffers DA, Eipper BA, Watson SJ (1992) Expression of peptidylglycine alpha-amidating monooxygenase (EC 1.14.17.3) in the rat central nervous system. J Neurosci 12:222–234
Sherman TG, Civelli O, Douglass J, Herbert E, Watson SJ (1986a) Coordinate expression of hypothalamic pro-dynorphin and pro-vasopressin mRNAs with osmotic stimulation. Neuroendocrinology 44:222–228
Sherman TG, McKelvy JF, Watson SJ (1986b) Vasopressin mRNA regulation in individual hypothalamic nuclei: a Northern and in situ hybridization analysis. J Neurosci 6:1685–1694
Sietzen M, Schober M, Fischer-Colbrie R, Scherman D, Sperk G, Winkler H (1987) Rat adrenal medulla: levels of chromogranins, enkephalins, dopamine β-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience 22:131–139
Stoffers DA, Green CB-R, Eipper BA (1989) Alternative mRNA splicing generates multiple forms of peptidyl-glycine alpha-amidating monooxygenase in rat atrium. Proc Natl Acad Sci USA 86:735–739
Thiele EA, Eipper BA (1990) Effect of secretagogues on components of the secretory system in AtT-20 cells. Endocrinology 126:809–817
Thiele EA, Marek KL, Eipper BA (1989) Tissue-specific regulation of peptidylglycine alpha-amidating monooxygenase expression. Endocrinology 125:2279–2288
Thompson ME, Zimmer WE, Wear LB, MacMillan LA, Thompson WJ, Huttner WB, Hidaka H, Scammell JG (1992) Differential regulation of chromogranin B/secretogranin I and secretogranin II by forskolin in PC12 cells. Mol Brain Res 12:195–202
Uhl GR, Zingg HH, Habener JF (1985) Vasopressin mRNA in situ hybridization: localization and regulation studied with oligonucleotide cDNA probes in normal and Brattleboro rat hypothalamus. Proc Natl Acad Sci USA 82:5555–5559
Van den Pol AN, Decavel C, Levi A, Paterson B (1989) Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci 9:4122–4137
Weiler R, Marksteiner J, Bellmann R, Wohlfarter T, Schober M, Fischer-Colbrie R, Sperk G, Winkler H (1990) Chromogranins in rat brain: characterization, topographical distribution and regulation of synthesis. Brain Res 532:87–94
Winkler H, Fischer-Colbrie R (1992) The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49:497–528
Winkler H, Apps DK, Fischer-Colbrie R (1986) The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience 18:261–290
Young WS III (1989) In situ hybridization. Histochemical detection of neuropeptide mRNA using DNA and RNA probes. Methods Enzymol 168:702–709
Young WS III, Horvath S, Palkovits M (1990) The influences of hyperosmolarity and synaptic inputs on galanin and vasopressin expression in the hypothalamus. Neuroscience 39:115–125
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mahata, S.K., Mahata, M., Fischer-Colbrie, R. et al. In situ hybridization: mRNA levels of secretogranin II, VGF and peptidylglycine alpha-amidating monooxygenase in brain of salt-loaded rats. Histochemistry 99, 287–293 (1993). https://doi.org/10.1007/BF00269101
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00269101