Summary
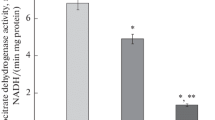
A new method for the histochemical demonstration of pyruvate kinase (PK) activity was developed using a semi-permeable membrane and ATP-dependent phosphorylation of glucose coupled with tetrazolium reduction via glucose-6-phosphate dehydrogenase (G6PD) in order to investigate normal liver tissue nd neoplastic hepatic nodules induced by thioacetamide (TAA). A series of control reactions and comparison with microbiochemical analysis of microdissected lyophilised material were used to determine the specificity of the reaction. In agreement with earlier reports, an activity gradient in control liver decreasing from zone 3 to zone 1 was apparent both histochemically and after biochemical analysis. Liver neoplastic nodules induced by 25 weeks dietary thioacetamide administration and characterized by increased G6PD demonstrated a clear decrease in PK activity. In contrast, epithelial cells within areas of cholangio-fibrosis thought to be direct precursors for cholangioccllular tumour development were characterized by a strong increase. Comparison of the results with immunohistochemical and biochemical data from the literature indicate that the specific histochemical method described will be of great assistance in future assessment of disease and physiological alteration in activity of this key enzyme of glycolysis.
Similar content being viewed by others
References
Bannasch P, Massner B (1976) Histogenese und Cytogenese von Cholangiofibromen und Cholangiocarcinomen bei Nitrosomorpholin-vergifteten Ratten. Z Krebsforsch 87: 239–255
Bannasch P, Zerban H (1987) Modulation of hepatocellular phenotype and proliferation in liver cirrhosis. In: Boyer JL, Bianchin L (eds) Liver cirrhosis. MTP Press, Lancaster Boston The Hague Dordrecht, pp 27–38
Bannasch P, Hesse J, Angerer H (1974) Hepathocelluläre Glycogenose und die Genese sogenannter hyperplastischer Knoten in der Thioacetamid-vergifteten Rattenleber. Virchows Arch B 17: 29–50
Bannasch P, Hacker HJ, Klimek F, Mayer D (1984) Hepatocellular glycogenosis and related pattern of enzymatic changes during hepatocarcinogenesis. Adv Enzyme Regul 22: 97–121
Chatzipanagiotou S, Nath A, Vogt B, Jungerimann K (1985) Alteration in the capacities as well as in the zonal and cellular distributions of pyruvate kinase L and M2 in regenerating rat liver. Biol Chem Hoppe Seyler 366: 271–280
Ehemann V, Mayer D, Hacker HJ, Bannasch P (1986) Loss of adenylate cyclase activity in preneoplastic and neoplastic lesions induced in rat liver by N-nitrosomorpholine. Carcinogenesis 7: 567–573
El-Maghrabi MR, Claus TH, McGrane MM, Pilkis SJ (1982) Influence of phosphorylation on the interaction of effectors with rat liver pyruvate kinase. J Biol Chem 257: 233–240
Engström L (1978) The regulation of liver pyruvate kinase by phosphorylation — dephosphorylation. Curr Top Cell Regul 13: 29–51
Farina FA, Shatton JB, Morris HP, Weinhouse S (1974) Isozymes of pyruvate kinase in liver and hepatomas of the rat. Cancer Res 34: 1439–1446
Fischer G, Domingo M, Lodder D, Katz N, Reinacher M, Eigenbrodt E (1987a) Immunohistochemical demonstration of decreased L-pyruvate kinase in enzyme altered rat liver lesions produced by different carcinogens. Virchows Arch B 53: 359–364
Fischer G, Ruschenburg I, Eigenbrodt E, Katz N (1987b) Decrease in glucokinase and glucose-6-phosphatase and increase in hexokinase in putative preneoplastic lesions of rat liver. J Cancer Res Clin Oncol 113: 430–436
Gali P (1979) Localisation épitheliade de la pyruvate kinase L (ATP pyruvate phosphotransférase E.C. 2.7.1.40) dans le foie de rat. C R Acad Sci Paris Série D 289: 1029–1032
Guder WG, Schmidt U (1976) Liver cell heterogeneity. The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase (GTP) in the liver lobule of fed and starved rats. Hoppe-Seyler's Z Physiol Chem 357: 1793–1800
Hacker HJ (1978) Histochemical demonstration of glycogen phosphorylase (E.C. 2.4.1.1) through the use of semi-permeable membranes. Histochemistry 58: 289–296
Hacker HJ, Moore MA, Mayer D, Bannasch P (1982) Correlative histochemistry of some enzymes of carbohydrate metabolism in preneoplastic and neoplastic lesions in the rat liver. Carcinogenesis 3: 1265–1272
Hall ER, Cottam GL (1978) Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem 9: 785–793
Jungermann K, Katz N (1982) Metabolic heterogeneity of liver parenchyma. In: Sies H (ed) Metabolic compartmentation. Academic Press, London New York, pp 411–436
Kew MC, Popper H (1984) Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis 4: 136–146
Klimek F, Mayer D, Bannasch P (1984) Biochemical microanalysis of glycogen content and glucose-6-phosphate dehydrogenase activity in focal lesions of the rat liver induced by N-nitrosomorpholine. Carcinogenesis 5: 265–268
Lawrence GM, Trayer IP, Walker DG (1984) Histochemical and immunohistochemical localization of hexokinase isoenzymes in normal rat liver. Histochem J 16: 1099–1111
Lienhard GE, Secemski II (1973) P, P5-Di(adenosine-5′)pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem 10: 1121–1123
Malvaldi G, Pollera M (1982) Long-term fate of the bile duct cells proliferated during chronic thioacetamide poisoning. Int J Tissue React 4: 55–61
Meijer AEFH, de Vries GP (1974) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. IV. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (decarboxylating). Histochemistry 40: 349–359
Middleton MC, Walker DG (1972) Comparison of the properties of two forms of pyruvate kinase in rat liver and determination of their separate activities during development. Biochem J 127: 721–731
Miethke H, Wittig B, Nath A, Zierz S, Jungermann K (1985) Metabolic zonation in liver of diabetic rats. Zonal distribution of phosphoenolpyruvate carboxykinase, pyruvate kinase, glucose-6-phosphatase and succinate dehydrogenase. Biol Chem Hoppe Seyler 366: 493–501
Munnich A, Marie J, Reach G, Vaulont S, Simon MP, Kahn A (1984) In vivo hormonal control of L-type pyruvate kinase gene expression. Effects of glucagon, cyclic AMP, insulin, cortisone and thyroid hormones on the dietary induction of mRNAs in the liver. J Biol Chem 259: 10228–10231
Noguchi T, Inoue H, Chen HL, Matsubara KI, Tanaka T (1983) Molecular cloning of DNA complementary to rat L-type pyruvate kinase mRNA. Nutritional and hormonal regulation of L-type pyruvate kinase mRNA concentration. J Biol Chem 258: 15220–15223
Nuber R, Teutsch HF, Sasse D (1980) Metabolic zonation of thioacetamide-induced liver cirrhosis. Histochemistry 69: 277–288
Rappaport AM (1973) The microcirculatory hepatic unit. Microvasc Res 6: 212–228
Reinacher M, Eigenbrodt E, Gerbracht U, Zeuk G, Timmermann-Trosiener J, Bentley P, Waechter F, Schulte-Hermann R (1986) Pyruvate kinase isoenzymes in altered foci and carcinoma of rat liver. Carcinogenesis 7: 1351–1358
Sachs L (1978) Angewandte Statistik, 5. Auflage, Springer, Berlin Heidelberg New York
Sato K, Takaya S, Imai F, Hatayama I, Ito N (1978) Different deviation patterns of carbohydrate metabolising enzymes in primary rat hepatomas induced by different chemical carcinogens. Cancer Res 38: 3086–3093
Seelmann-Eggebert G, Mayer D, Mecke D, Bannasch P (1987) Expression and regulation of glycogen phosphorylase in preneoplastic and neoplastic hepatic lesions in rats. Virchows arch B 53: 44–51
Teutsch HF (1978) Quantitative determination of G6Pase activity in histochemically defind zones of the liver acinus. Histochemistry 58: 281–288
Thamavit W, Kongkanuntn R, Tiwawech D, Moore MA (1987) Level of Opisthorchis infestation and carcinogen dose-dependence of cholangio-carcinoma induction in Syrian golden hamsters. Virchows Arch B 54: 52–58
Van Berkel JC, Koster JF, Hülsmann WC (1972) Distribution of L- and M-type pyruvate kinase between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta 276: 425–429
Vaulont S, Munnich A, Decaux JF, Kahn A (1986) Transcriptional and post-transcriptional regulation of L-type pyruvate kinase gene expression in rat liver. J Biol Chem 261: 7621–7625
Yanagi S, Makiura S, Aroi M, Matsumara K, Hirao K, Ito N, Tanaka T (1974) Isozyme patterns of pyruvate kinase in various primary liver tumors induced during the process of hepatocarcinogenesis. Cancer Res 34: 2283–2289
Yanagi S, Tsuda H, Sakamoto M, Ninomiya Y, Ito N (1984) Evaluation of a histologic classification of mouse liver tumors based on pyruvate kinase isozymes and status of host lipids. J Natl Cancer Inst 73: 1311–1319
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft. Dr. Malcolm A. Moore is the recipient of a guest research scientist fellowship from the German Cancer Research Center
Rights and permissions
About this article
Cite this article
Klimek, F., Moore, M.A., Schneider, E. et al. Histochemical and microbiochemical demonstration of reduced pyruvate kinase activity in thioacetamide-induced neoplastic nodules of rat liver. Histochemistry 90, 37–42 (1988). https://doi.org/10.1007/BF00495704
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00495704