Abstract
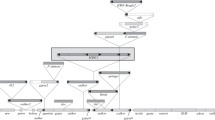
Phylogenetic studies suggest that mobile element families are unstable components of the Drosophila genome. Two examples of immobilization of a transposable element family are presented here: as judged by their constant genomic organization among unrelated strains, the F and I element families have been respectively immobilized for a long time in D. simulans and in the reactive D. melanogaster strains (these are the laboratory strains which escaped the recent I invasion of D. melanogaster natural populations). All the elements of these defective families are located in the β heterochromatic portion of the genome. Moreover, most if not all of the β heterochromatic sequences into which the defective I elements are embedded are themselves non-mobile members of various nomadic families such as mdg 4, 297, 1731, F and Doc. These results are discussed with special emphasis on the possible nomadic origin of β heterochromatin components and on the mechanisms of evolutionary turnover of the transposable element families.
Similar content being viewed by others
References
Ananiev EV, Barsky VE, Ilyin YV, Rysic MV (1984) The arrangement of transposable elements in the polytene chromosomes of Drosophila melanogaster. Chromosoma 90:366–377
Appleyard RK (1954) Segregation of new lysogenic types during growth of a doubly lysogenic strain derived from E. coli K12. Genetics 39:440–452
Bayev AA Jr, Lyubomirskaya NV, Dzhumagaliev EB, Ananiev EV, Amiantova IG, Ilyin YV (1984) Structural organisation of transposable element mdg4 from D. melanogaster and a nucleotide sequence of its long terminal repeats. Nucleic Acid Res 12:3707–3723
Blackman RK, Grimaila R, Koehler MMD, Gelbart WM (1987) Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in D. melanogaster. Cell 49:497–505
Bregliano JC, Kidwell MG (1983) Hybrid dysgenesis determinants. In: Shapiro JA (ed) Mobile genetic elements. Academic Press, London New York, pp 363–410
Bucheton A, Paro R, Sang HM, Pelisson A, Finnegan DJ (1984) The molecular basis of IR hybrid dysgenesis in D. melanogaster: identification, cloning and properties of the I factor. Cell 38:153–163
Coyne JA (1986) Meiotic segregation and male recombination in interspecific hybrids of Drosophila. Genetics 114:485–494
Crozatier M, Vaury C, Busseau I, Pelisson A, Bucheton A (1988) Structure and genomic organization of I elements in I-R hybrid dysgenesis in Drosophila melanogaster. Nucleic Acids Res 16:9199–9213
Daniels SB, Strausbaugh LD (1986) The distribution of P. element sequences in Drosophila: The willistoni and saltans species groups. J Mol Evol 23:138–148
Dawid IB, Long EO, Di Nocera PP, Pardue ML (1981) Ribosomal insertion-like elements in Drosophila melanogaster are interspersed with mobile sequences. Cell 25:399–408
Di Nocera PP (1988) Close relationship between non LTR retroposons in D. melanogaster. Nucleic Acids Res 16:4041–4052
Di Nocera PP, Dawid IB (1983) Interdigitated arrangement of two oligo-A terminated DNA sequences in Drosophila. Nucleic Acids Res 11:5475–5482
Di Nocera PP, Digan ME, Dawid IB (1983) A family of oligoadenylate-terminated transposable sequences in D. tmelanogaster. J Mol Biol 168:715–727
Di Nocera PP, Graziani F, Lavorgna G (1986) Genomic and structural organization of D. melanogaster G elements. Nucleic Acid Res 14:675–691
Dowset AP (1983) Closely related species of Drosophila can contain different libraries of middle repetitive DNA sequences. Chromosoma 88:104–108
Dowset AP, Young MW (1982) Differing levels of dispersed repetitive DNA among closely related species of Drosophila. Proc Natl Acad Sci USA 79:4570–4574
Dunsmuir P, Brorein WJ Jr, Simon MA, Rubin GM (1980) Insertion of the Drosophila transposable element copia generates a 5 base pair duplication. Cell 21:575–579
Engels WR (1989) P elements in Drosophila species. In: Berg DE, Howe MM (eds) Mobile DNA. American Society for Microbiology, Washington, in press
Engels WR, Preston CR (1979) Hybrid dysgenesis in Drosophila melanogaster: The biology of male and female sterility. Genetics 92:111–128
Fannig T, Singer M (1987) The LINE-I DNA sequences in four mammalian orders predict proteins that conserve homologies to retrovirus proteins. Nucleic Acids Res 15:2251–2260
Finnegan DJ (1989) The I factor and I-R hybrid dysgenesis in D. melanogaster. In: Berg DE, Howe MM (eds) Mobile DNA. American Society for Microbiology, Washington, in press
Gall JG, Cohen EH, Polan ML (1971) Repetitive DNA sequences in Drosophila. Chromosoma 33:319–344
Goldberg ML, Paro R, Gehring WJ (1982) Molecular cloning of the white locus region of D. melanogaster using a large transposable element. EMBO J 1:93–98
Gough JA, Murray NE (1983) Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol 166:1–19
Heitz E (1934) Über α- und β-heterochromatin sowie Konstanz und Bau der Chromomeren bei Drosophila. Biol Zentralb 54:588–609
Hunt JA, Bishop III JG, Carson HL (1984) Chromosomal mapping of a middle-repetitive DNA sequence in a cluster of five species of Hawaiian Drosophila. Proc Natl Acad Sci USA 81:7146–7150
Ilyin YV, Chmeliauskaite VG, Georgiev GP (1980) Double stranded sequences in RNA of D. melanogaster: relation to mobile dispersed genes. Nucleic Acid Res 8:3439–3457
Kaplan N, Darden T, Langley CH (1985) Evolution and extinction of transposable elements in mendelian populations. Genetics 109:459–480
Karn J, Brenner S, Barnett L, Cesaerini C (1980) Novel bacteriophage cloning vector. Proc Natl Acad Sci USA 77:5172–5176
Kidd SJ, Glover DM (1980) A DNA segment from D. melanogaster which contains five tandemly repeating units homologous to the major λ DNA insertion. Cell 19:103–119
Kidwell MG (1983) Evolution of hybrid dysgenesis determinants in D. melanogaster. Proc Natl Acad Sci USA 80:1655–1659
Kidwell MG, Kidwell JF (1975) Cytoplasm chromosome interaction in D. melanogaster. Nature 253:755–756
Kidwell MG, Kidwell JF, Sved JA (1977) Hybrid dysgenesis in D. melanogaster: a syndrome of aberrant traits including mutation, sterility and male recombination. Genetics 86:813–833
Kimmel BE, Maiyoi OK, Young JR (1987) Ingi a 5.2 kb dispersed sequence element from Trypanasoma brucei that carries half of a smaller mobile element at either end and has homology with mammalian LINEs. Mol Cell Biol 7:1465–1475
Kugimiya W, Ikenaga H, Saigo K (1983) Close relationship between the long terminal repeats of avian leukosis-sarcoma virus and copia-like movable genetic elements of Drosophila. Proc Natl Acad Sci USA 80:3193–3197
Lansman RA, Stacey SN, Grigliatti TA, Brock HW (1985) Sequences homologous to the P mobile element of Drosophila melanogaster are widely distributed in the subgenus Sophophora. Nature 318:561–563
Lansman RA, Shade RO, Grigliatti TA, Brock HW (1987) Evolution of P transposable elements: Sequences of Drosophila nebulosa P elements. Proc Natl Acad Sci USA 84:6491–6495
Lifschytz E (1978) Fine structure analysis and genetic organization at the base of the X chromosome in Drosophila melanogaster. Genetics 88:457–467
Lifton RP, Goldberg ML, Karp RW, Hogness DS (1977) The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harbor Symp Quant Biol 42:1047–1051
Long EO, Dawid IB (1979) Expression of ribosomal DNA insertions in D. melanogaster. Cell 18:1185–1196
Lueders KK (1987) Specific association between type-II intracisternal A-particle elements and other repetitive sequences in the mouse genome. Gene 52:139–146
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, NY
Martin G, Wiernasz D, Schedl P (1983) Evolution of Drosophila repetitive-dispersed DNA. J Mol Evol 19:203–213
Micard D, Couderc JL, Sobrier ML, Giraud G, Dastugue B (1988) Molecular study of the retrovirus-like transposable element 412, a 20-OH ecdysone responsive repetitive sequence in Drosophila cultured cells. Nucleic Acid Res 16:455–470
Miklos GLG, Yamamoto MT, Davies J, Pirrotta V (1988) Microcloning reveals a high frequency of repetitive sequences characteristic of chromosome 4 and the β-heterochromatin of Drosophila melanogaster. Proc Natl Acad Sci USA 85:2051–2055
Mizrokhi LJ, Obolenkova LA, Priimägi AF, Ilyin YV, Gerasimova TI, Georgiev GP (1985) The nature of unstable insertion mutations and reversions in the locus cut of D. melanogaster: molecular mechanism of transposition memory. EMBO J 4:3781–3787
Mizrokhi LJ, Georgieva SG, Ilyin YV (1988) Jockey, a mobile Drosophila element similar to mammalian LINEs, is transcribed from the internal promoter by RNA polymerase II. Cell 54:685–691
Montgomery E, Charlesworth B, Langley CH (1987) A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet Res 49:31–41
Pardue ML (1975) Repeated DNA sequences in the chromosomes of higher organisms. Genetics 79:159–170
Pardue ML, Dawid I (1981) Chromosomal locations of two DNA segments that flank ribosomal insertion-like sequences in Drosophila: Flanking sequences are mobile elements. Chromosoma 83:29–43
Paro R, Goldberg ML, Gehring WJ (1983) Molecular analysis of large transposable elements carrying the white locus of D. melanogaster EMBO J 2:853–860
Pelisson A, Bregliano JC (1981) The IR system of hybrid dysgenesis in D. melanogaster: construction and characterization of a noninducer stock. Biol Cell 40:159–164
Peronnet F, Becker JL, Becker J, d'Auriol L, Galibert F, Best-Belpomme M (1986) 1731, a new retrotransposon with hormone modulated expression. Nucleic Acid Res 14:9017–9033
Picard G, L'Heritier P (1971) A maternally inherited factor inducing sterility in D. melanogaster. Dros Inf Serv 46:54
Pont G, Degroote F, Picard G (1987) Some extrachromosomal circular DNAs from Drosophila embryos are homologous to tandemly repeated genes. J Mol Biol 195:447–451
Rose MR, Doolittle WF (1983) Molecular biological mechanisms of speciation. Science 220:157–162
Scherer G, Tschudi C, Perera S, Delvis H, Pirotta V (1982) B104 a new dispersed repeated gene family in Drosophila melanogaster and its analogies with retroviruses. J Mol Biol 157:435–452
Schwartz-Sommer Z, Leclercq L, Göbel E, Saedler H (1987) Cin4, an insert altering the structure of the A1 gene in Zea mays, exhibits properties of non viral retrotransposons. EMBO J 6:3873–3880
Simonelig M, Bazin C, Pelisson A, Bucheton A (1988) Transposable and non transposable elements similar to the I factor involved in I-R hybrid dysgenesis in D. melanogaster coexist in various Drosophila species. Proc Natl Acad Sci USA 85:1141–1145
Stacey SN, Lansman RA, Brock HW, Grigliatti TA (1986) Distribution and conservation of mobile elements in the genus Drosophila. Mol Biol Evol 36:522–534
Steffenson DM, Appels R, Peacock WJ (1981) Distribution of two highly repeated DNA sequences within Drosophila melanogaster chromosomes. Chromosoma 83:525–541
Wensink PC, Tabata S, Pachl C (1979) The clustered and scrambled arrangement of moderately repetitive elements in Drosophila DNA. Cell 18:1231–1246
Will BM, Bayev AA, Finnegan DJ (1981) Nucleotide sequence of terminal repeats of 412 transposable elements of D. melanogaster. J Mol Biol 153:897–915
Yannopoulos G, Stamatis N, Monastirioti M, Hatzopoulos P, Louis K (1987) Hobo is responsible for the induction of hybrid dysgenesis by strains of D. melanogaster bearing the male recombination factor 23.5 MRF. Cell 49:487–495
Young MW (1979) Middle repetitive DNA: a fluid component of the Drosophila genome. Proc Natl Acad Sci USA 76:6274–6278
Young BS, Pession A, Traverse KL, French C, Pardue ML (1983) Telomere regions in Drosophila share complex DNA sequence with pericentric heterochromatin. Cell 34:85–94
Zelentsova ES, Vashakidze RP, Krayev AS, Evgen'ev MB (1986) Dispersed repeats in Drosophila virilis: elements mobilized by interspecific hybridization. Chromosoma 93:469–476
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vaury, C., Bucheton, A. & Pelisson, A. The β heterochromatic sequences flanking the I elements are themselves defective transposable elements. Chromosoma 98, 215–224 (1989). https://doi.org/10.1007/BF00329686
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00329686