Abstract
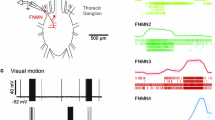
InManduca sexta, large tangential cells connect the medulla via the lobula valley (LoV) tract to the midbrain and the contralateral medulla. Tract neurons have been stained and recorded to determine their responses to optomotor stimulation. Neurons in the LoV-tract comprise a physiologically and anatomically heterogeneous population:
-
1.
Motion insensitive medulla tangential (Mt) neurons arise from cell bodies in the ventral rind. Heterolateral cells arborize massively in both medullae and one or both halves of the midbrain. Mt-neurons respond to changes in light intensity. Physiological and anatomical evidence argues for their monocularity and transmission from the medulla on the side of the soma to the central brain and the contralateral medulla.
-
2.
Motion sensitive neurons with cell bodies behind the protocerebral bridge connect the midbrain to the ipsior contralateral medulla. Direction-selective responses are characterized by excitation to motion in the preferred and inhibition in the opposite direction with maxima either in a horizontal or vertical direction. Peak values appear at contrast frequencies of appr. 3/s. The results suggest that these neurons are binocular and relay information from the midbrain to the medulla. They have been labelled as centrifugal medulla tangential (cMt) neurons.
The possible roles for tract neurons in visually guided behaviour are discussed.
Similar content being viewed by others
Abbreviations
- LoV:
-
lobula valley
- Mt:
-
medulla tangential
- cMt:
-
centrifugal medulla tangential
- sMt:
-
small medulla tangential
- Tmt:
-
transmedullary tangential
References
Baumhover AH, Cantelo WW, Hobgood JM, Knott CM, Lam JJ (1977) An improved method for mass rearing the tobacco hornworm. Agricultural Research Service, USDA ARS-5-167, US Government Printing Office, Washington DC
Bausenwein B, Fischbach KF (1992) Activity labeling patterns in the medulla ofDrosophila melanogaster caused by motion stimuli. Cell Tissue Res 270:25–35
Bennett RR, White RH (1989) Influence of carotenoid deficiency on visual sensitivity, visual pigment and P-face particles of photoreceptor membrane in the mothManduca sexta. J Comp Physiol A 164:321–331
Blest AD, Collett TS (1965) Microelectrode studies of the medial protocerebrum of some Lepidoptera. I. Responses to simple binocular visual stimulation. J Insect Physiol 11:1079–1103
Buchner E (1984) Behavioral analysis of spatial vision in insects. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum New York London, pp 561–621
Buchner E, Buchner S, Bülthoff I (1984) Deoxyglucose mapping of nervous activity induced inDrosophila brain by visual movement. I. Wildtype. J Comp Physiol A 155:471–483
Cajal SR, Sánchez D (1915) Contribución al conocimiento de los céntros nerviosos de los inséctos. Parte I, rétina y centros ópticos. Trab Lab Invest Biol Univ Madrid 13:1–168
Campos-Ortega JA, Strausfeld NJ (1972) Columns and layers in the second synaptic region of the fly's visual system. The case for two superimposed neuronal architectures. In: Wehner R (ed) Information processing in the visual system of arthropods. Springer, Berlin Heidelberg New York, pp 31–36
Collett TS (1970) Centripetal and centrifugal visual cells in the medulla of the insect optic lobe. J Neurophysiol 33:239–256
Collett TS, Blest DA (1966) Binocular, directionally selective neurones, possibly involved in the optomotor response of insects. Nature 212:1330–1333
DeVoe RD, Ockleford EM (1976) Intracellular responses from cells of the medulla of the fly,Calliphora erythrocephala. Biol Cybern 23:13–24
Dombrowski UJ (1991) Untersuchungen zur funktionellen Organisation des Flugsystems vonManduca sexta (L.). Doct Dissert, University of Cologne
Dombrowski UJ, Milde JJ, Wendler G (1990) Visual control of compensatory head movements in the sphinx moth. In: Gribakin FG, Wiese K, Popov AV (eds.) Sensory systems and communication in arthropods. Birkhäuser, Basel Boston Berlin, pp 127–133
Egelhaaf M (1985) On the neuronal basis of figure-ground discrimination by relative motion in the visual system of the fly. II: Figure-detection cells, a new class of visual interneurons. Biol Cybern 52:195–208
Egelhaaf M, Borst A, Reichardt W (1989) Computational structure of a biological motion detection system as revealed by local detector analysis in the fly's nervous system. J Opt Soc Am A 6:1070–1087
Fischbach KF, Dittrich APM (1989) The optic lobe ofDrosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res 258:441–475
Gilbert C, Strausfeld NJ (1991) The functional organization of male-specific visual neurons in flies. J Comp Physiol A 169:395–411
Gilbert C, Penisten DK, DeVoe RD (1991) Discrimination of visual motion from flicker by identified neurons in the medulla of the fleshflySarcophaga bullata. J Comp Physiol A 168:653–673
Hassenstein B, Reichardt W (1956) Systemtheoretische Analyse der Zeit-, Reihenfolgen- und Vorzeichenauswertung bei der Bewegungsperzeption des RüsselkäfersChlorophanus. Z Naturforsch 11b:513–524
Hausen K (1984) The lobula-complex of the fly: Structure, function and significance in visual behavior. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum, New York London, pp 523–559
Hausen K, Egelhaaf M (1989) Neural mechanisms of visual course control in insects. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Heidelberg New York, pp 391–424
Hertel H, Maronde U (1987) The physiology and morphology of centrally projecting visual interneurones in the honeybee brain. J Exp Biol 133:301–315
Hertel H, Schäfer S, Maronde U (1987) The physiology and morphology of visual commissures in the honeybee brain. J Exp Biol 133:283–300
Homberg U, Hildebrand JG (1989a) Serotonin-immunoreactive neurons in the median protocerebrum and suboesophageal ganglion of the sphinx mothManduca sexta. Cell Tissue Res 258:1–24
Homberg U, Hildebrand JG (1989b) Serotonin-immunoreactivity in the optic lobes of the sphinx mothManduca sexta and colocalization with FMRFamide- and SCPB-immunoreactivity. J Comp Neurol 288:243–253
Homberg U, Kingan TG, Hildebrand JG (1987) Immunocytochemistry of GABA in the brain and suboesophageal ganglion ofManduca sexta. Cell Tissue Res 248:1–24
Ibbotson MR, Maddess T, DuBois R (1991) A system of insect neurons sensitive to horizontal and vertical image motion connects the medulla and midbrain. J Comp Physiol A 169:355–367
Itagaki H, Hildebrand JG (1990) Olfactory interneurons in the brain of the larval sphinx mothManduca sexta. J Comp Physiol A 167:309–320
Kelly KM, Mote MI (1990) Electrophysiology and anatomy of medulla interneurons in the optic lobe of the cockroachPeriplaneta americana. J Comp Physiol A 167:745–756
Maddess T, Dubois RA, Ibbotson MR (1991) Response properties and adaptation of neurones sensitive to image motion in the butterflyPapilio aegeus. J Exp Biol 161:171–199
Milde JJ (1989) Neural basis of head movements evoked by optomotor stimuli in the mothManduca sexta. Soc Neurosci Abstr 15:1290
Milde JJ (1991) The role of brain interneurons underlying visual behaviour as revealed by simultaneous recorded muscle activity. In: Elsner N, Penzlin H (eds) Synapse—transmission—modulation. Proc 19th Göttingen Neurobiol Conf, Thieme, Stuttgart New York, p 271
Milde JJ, Strausfeld NJ (1990) Cluster organization and response characteristics of the giant fibre pathway of the blowflyCalliphora erythrocephala. J Comp Neurol 294:59–75
Milde JJ, Seyan HS, Strausfeld NJ (1987) The neck motor system of the flyCalliphora erythrocephala. II. Sensory organization. J Comp Physiol A 160:225–238
Milde JJ, Gronenberg W, Strausfeld NJ (1992) The head-neck system of the blowflyCalliphora: functional organization and comparisons with the sphinx mothManduca sexta. In: Berthoz A, Graf W, Vidal PP (eds) The head-neck sensory motor system. Oxford University Press, New York Oxford, pp 64–70
Mimura K (1971) Movement discrimination by the visual system of flies. Z Vergl Physiol 73:105–138
O'Shea M, Williams JLD (1974) The anatomy and output connection of a locust visual interneurone; the lobula giant movement detector (LGMD) neurone. J Comp Physiol 91:257–266
Osorio D (1986) Directionally selective cells in the locust medulla. J Comp Physiol A 159:841–847
Reichardt W (1957) Autokorrelations-Auswertung als Funktionsprinzip des Zentralnervensystems. Z Naturforsch 12b:448–457
Rind FC (1987) Non-directional, movement sensitive neurones of the locust optic lobe. J Comp Physiol A 161:477–494
Rowell CHF, Reichert H (1986) Three descending interneurons reporting deviation from course in the locust. II. Physiology. J Comp Physiol A 158:775–794
Rowell CHF, O'Shea M, Williams JLD (1977) The neuronal basis of a sensory analyser, the acridid movement detector system. IV. The preference for small field stimuli. J Exp Biol 68:157–185
Scharstein H (1989) A universal projector for optomotor stimulation. In: Elsner N, Singer W (eds) Dynamics and plasticity in neuronal systems. Proc 17th Göttingen Neurobiol Conf, Thieme, Stuttgart New York, p 116
Spurr AR (1969) A low viscosity embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Strausfeld NJ (1970) Golgi studies on insects. Part II. The optic lobes of Diptera. Phil Trans R Soc London B 258:135–223
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strausfeld NJ (1989) Beneath the compound eye: neuroanatomical analysis and physiological correlates in the study of insect vision. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Heidelberg New York, pp 317–359
Strausfeld NJ, Blest AD (1970) Golgi studies on insects. Part I. The optic lobes of Lepidoptera. Phil Trans R Soc London B 258:81–134
Strausfeld NJ, Seyan HS (1985) Convergence of visual, haltere and prosternal inputs at neck motor neurons ofCalliphora erythrocephala. Cell Tissue Res 240:601–615
Swihart SL (1968) Single unit activity in the visual pathway of the butterflyHeliconius erato. J Insect Physiol 14:1589–1601
Wehner R (1981) Spatial vision in insects. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6C. springer, Berlin Heidelberg New York, pp 287–616
Wendler G, Müller M, Dombrowski U (1993) The activity of pleurodorsal muscles during flight and rest in the mothManduca sexta (L.). J Comp Physiol A 173:65–75
Williams JLD (1975) Anatomical studies of the insect central nervous system, a ground-plan of the mid-brain and an introduction to the central complex in the locustSchistocerca gregaria (Orthoptera). J Zool (Lond) 176:67–86
Willis MA, Arbas EA (1991) Odor-modulated upwind flight of the sphinx moth,Manduca sexta L. J Comp Physiol A 169:427–440
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Milde, J.J. Tangential medulla neurons in the mothManduca sexta. Structure and responses to optomotor stimuli. J. Comp. Physiol. 173, 783–799 (1993). https://doi.org/10.1007/BF02451909
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02451909