Summary
-
1.
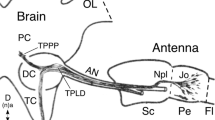
The cerci of locusts are paired, unsegmented, cone-shaped structures arising from a depression on either side of the tip of the abdomen (Fig. 2).
-
2.
The cerci bear hair sensilla of two basic types (Fig. 3): (a) filiform hairs, which emerge from a cuticular pit, are flexibly mounted, and are sensitive to wind and to mechanical displacement; and (b) bristle (or trichoid) hairs, which originate directly from the surface of the cercus, and are less flexibly mounted. The axons of neurones innervating these hairs group together into successively larger bundles before joining the cercal nerve which runs to the terminal ganglion (Fig. 4).
-
3.
In the terminal ganglion the majority of cercal afferents (Fig. 5), and all identified filiform afferents (Fig. 6) end in contact with a cercal glomerulus formed by the densely interwoven arborizations of giant and non-giant interneurones (Figs. 5, 7,9).
-
4.
A transverse section of the ventral nerve cord anterior to the terminal ganglion reveals four axons, one medial and three grouped together dorsolaterally, with distinctly larger profiles than all the others (Figs. 8, 9). The somata associated with these axons are located contralaterally in the terminal ganglion. All four interneurones have at least some projections into both cercal glomeruli. The soma of giant interneurone 1 (GIN 1), which is associated with the large medial axon, lies laterally in neuromere 9, the somata of GINs 2–4 are located ventrally in neuromere 9, ventrally in neuromere 10, and postero-dorsally in neuromere 11, respectively (Figs. 7,9).
-
5.
All four GINs run the length of the ventral nerve cord (Figs. 10, 11, 12) and end in the brain (Fig. 11). The axon of GIN 1 is found in the ventral intermediate tract (VIT), those of GINs 2, 3, 4 are initially in the lateral dorsal tract (LDT) but they cross to the dorsal intermediate tract (DIT) at the level of the most anterior free abdominal ganglion. All have medial branches in the abdominal ganglia (Figs. 10, 12). GIN 1 has a lateral branch (sometimes absent) only in the metathoracic ganglion, while the other GINs have lateral branches in all of the thoracic ganglia (Figs. 10, 11, 12).
-
6.
The cercal receptor/giant interneurone system ofLocusta is compared to those of other orthopteroid insects, with special attention to the origin and evolution of giant interneurones.
Similar content being viewed by others
References
Altman J (1983) Sensory inputs and the generation of the locust flight motor pattern: from the past to the future. In: Nachtigall W (ed) Biona Report 2. Gustav Fischer, Stuttgart, pp 127–136
Altman JS, Tyrer NM (1980) Filling selected neurons with cobalt through cut axons. In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques: insect nervous system. Springer, New York Heidelberg Berlin, pp 373–402
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res 138:359–363
Bacon JP, Murphey RK (1984) Receptive fields of cricket (Acheta domesticus) interneurones are related to their dendritic structure. J Physiol 352:601–623
Ball EE, Stone RC (1982) The cercal receptor system of the praying mantid,Archimantis brunneriana Sauss. I. Cercal morphology and receptor types. Cell Tissue Res 224:55–70
Ball EE, Boyan GS, Stone RC (1982) The cercal receptor system of the praying mantid,Archimantis brunneriana Sauss. II. Cercal nerve structure and projection and electrophysiological responses of individual receptors. Cell Tissue Res 224:71–80
Blagburn JM, Sattelle DB (1987) Presynaptic depolarization mediates presynaptic inhibition at a synapse between an identified mechanosensory neurone and giant interneurone 3 in the first instar cockroach,Periplaneta americana. J Exp Biol 127:135–157
Blagburn JM, Beadle DJ, Sattelle DB (1986) Differential synaptic input of filiform hair sensory neurones onto giant interneurones in the first-instar cockroach. J Insect Physiol 32:591–595
Boeckh J, Ernst K-D (1987) Contribution of single unit analysis in insects to an understanding of olfactory function. J Comp Physiol A 161:549–565
Boyan GS (1983) Postembryonic development in the auditory system of the locust. J Comp Physiol 151:499–513
Boyan GS (1988) Presynaptic inhibition of identified wind-sensitive afferents in the cercal system of the locust. J Neurosci 8:2748–2757
Boyan GS, Ball EE (1986) Wind-sensitive interneurones in the terminal ganglion of praying mantids. J Comp Physiol A 159:773–789
Boyan GS, Ball EE (1989a) Parallel inputs shape the response of a giant interneurone in the cercal system of the locust. J Insect Physiol 35:305–312
Boyan GS, Ball EE (1989b) The wind-sensitive cercal receptor/giant interneurone system of the locust,Locusta migratoria. II. Physiology of giant interneurones. J Comp Physiol A 165:511–521
Boyan GS, Ball EE (1989c) The wind-sensitive cercal receptor/giant interneurone system of the locust,Locusta migratoria. III. Cercal activation of thoracic motor pathways. J Comp Physiol A 165:523–537
Boyan GS, Ashman S, Ball EE (1986) Initiation and modulation of flight by a single giant interneuron in the cercal system of the locust. Naturwissenschaften 73:272–274
Boyan GS, Williams JLD, Ball EE (1989) The wind-sensitive cercal receptor/giant interneurone system of the locust,Locusta migratoria. IV. The non-giant interneurones. J Comp Physiol A 165:539–552
Callec J (1974) Synaptic transmission in the central nervous system of insects. In: Treherne JE (ed) Insect neurobiology. North-Holland, Amsterdam, pp 119–185
Camhi JM (1980) The escape system of the cockroach. Sci Am 243:144–157
Camhi JM (1984) Neuroethology: nerve cells and the natural behavior of animals. Sinauer, Sunderland
Collin SP (1985) The central morphology of the giant interneurons and their spatial relationship with the thoracic motorneurons in the cockroach,Periplaneta americana (Insecta). J Neurobiol 16:249–267
Cook PM (1951) Observations on giant fibres of the nervous system ofLocusta migratoria. Q J Micros Sci 92:297–305
Daley DL, Vardi N, Appignani B, Camhi JM (1981) Morphology of the giant interneurons and cercal nerve projections of the American cockroach. J Comp Neurol 196:41–52
Doe CQ, Goodman CS (1985) Early events in insect neurogenesis. I. Development and segmental differences in the pattern of neuronal precursor cells. Dev Biol 111:193–205
Edwards JS, Mann D (1981) The structure of the cercal sensory system and ventral nerve cord ofGrylloblatta. A comparative study. Cell Tissue Res 217:177–188
Edwards JS, Palka J (1974) The cerci and abdominal giant fibres of the house cricketAcheta domesticus. I. Anatomy and physiology of normal adults. Proc R Soc Lond B 185:83–103
Edwards JS, Reddy GJ (1986) Mechanosensory appendages and giant interneurons in the firebrat (Thermobia domestica, Thysanura): a prototype system for terrestrial predator evasion. J Comp Neurol 243:535–546
Edwards JS, Williams L (1981) Anterior-most projections of giant interneurones inAcheta domesticus terminate in mechano-receptor neuropile of the brain. Soc Neurosci Abstr 7:252
Fuller H, Ernst A (1977) Die Ultrastruktur der cercalen Cuticularsensillen vonPeriplaneta americana (L.). Zool Jb Anat 98:544–571
Gnatzy W (1976) The ultrastructure of the thread-hairs on the cerci of the cockroachPeriplaneta americana L.: the intermoult phase. J Ultrastruct Res 54:124–134
Gnatzy W, Schmidt K (1971) Die Feinstruktur der Sinneshaare auf den Cerci vonGryllus bimaculatus Degx. (Saltatoria, Gryllidae). I. Faden- und Keulenhaare. Z Zellforsch 122:190–209
Hartman HB, Bennett LP, Moulton BA (1987) Anatomy of equilibrium receptors and cerci of the burrowing desert cockroachArenavaga (Insecta, Blattodea). Zoomorphology 107:81–87
Hedwig B (1986) On the role in stridulation of plurisegmental interneurons of the acridid grasshopperOmocestus viridulus L. II. Anatomy and physiology of ascending and T-shaped interneurons. J Comp Physiol A 158:429–444
Heusslein R, Gnatzy W (1987) Central projections of campaniform sensilla on the cerci of crickets and cockroaches. Cell Tissue Res 247:591–598
Hoyle G (1958) The leap of the grasshopper. Sci Am 198:30–35
Hue B (1983) Electrophysiologie et pharmacologie de la transmission synaptique dans le système nerveux central de la blatte,Periplaneta americana L. PhD thesis, University of Angers
Jacobs GA, Miller JP (1985) Functional properties of individual neuronal branches isolated in situ by laser photoinactivation. Science 228:344–346
Jacobs GA, Murphey RK (1987) Segmental origins of the cricket giant interneuron system. J Comp Neurol 265:145–157
Jacobs GA, Miller JP, Murphey RK (1986) Integrative mechanisms controlling directional sensitivity of an identified sensory interneuron. J Neurosci 6:2298–2311
Kirk MD (1985) Presynaptic inhibition in the crayfish CNS: pathways and synaptic mechanisms. J Neurophysiol 54:1305–1325
Krenz WD, Reichert H (1985) Lateralized inhibitory input to an identified nonspiking local interneuron in the crayfish mechanosensory system. J Comp Physiol A 157:499–508
Levine RB, Murphey RK (1980) Pre- and postsynaptic inhibition of identified giant interneurons in the cricket (Acheta domesticus). J Comp Physiol 135:269–282
Mendenhall B, Murphey RK (1974) The morphology of cricket giant interneurons. J Neurobiol 5:565–580
Murphey RK (1981) The structure and development of a somatotopic map in crickets: the cercal afferent projection. Dev Biol 88:236–246
Murphey RK (1985) A second cricket cercal sensory system: bristle hairs and the interneurons they activate. J Comp Physiol A 156:357–367
O'Shea M, Williams JLD (1974) The anatomy and output connection of a locust visual interneurone; the Lobula Giant Movement Detector (LGMD) neurone. J Comp Physiol 91:257–266
O'Shea M, Rowell CHF, Williams JLD (1974) The anatomy of a locust visual interneurone; the descending contralateral movement detector. J Exp Biol 60:1–12
Palka J, Levine R, Schubiger M (1977) The cercus-to-giant interneuron system of crickets. J Comp Physiol 119:267–283
Potente A (1975) Untersuchungen zur Morphologie der cercalen Riesenfasern im Bauchmark vonLocusta migratoria. Hausarbeit, Ruhr-Universität, Bochum, FRG, unpublished
Reichert H, Plummer MR, Hagiwara G, Roth RL, Wine JJ (1982) Local interneurons in the terminal abdominal ganglion of the crayfish. J Comp Physiol 149:145–162
Reichert H, Plummer MR, Wine JJ (1983) Identified nonspiking local interneurons mediate nonrecurrent, lateral inhibition of crayfish mechanosensory interneurons. J Comp Physiol 151:261–276
Ritzmann RE (1984) The cockroach escape response. In: Eaton RC (ed) Neural mechanisms of startle behavior. Plenum, New York London, pp 93–131
Ritzmann RE, Camhi JM (1978) Excitation of leg motor neurons by giant interneurons in the cockroachPeriplaneta americana. J Comp Physiol 125:305–316
Ritzmann RE, Tobias ML, Fourtner CR (1980) Flight activity initiated via giant interneurons of the cockroach: evidence for bifunctional trigger interneurons. Science 210:443–445
Ritzmann RE, Pollack AJ, Tobias ML (1982) Flight activity mediated by intracellular stimulation of dorsal giant interneurons of the cockroachPeriplaneta americana. J Comp Physiol 147:313–322
Roonwal ML (1937) Studies on the embryology of the African migratory locust,Locusta migratoria migratorioides Reiche and Frm. (Orthoptera, Acrididae). II. Organogeny. Phil Trans R Soc Lond B 227:157–244
Rozhkova GI, Rodionova HI, Popov AV (1984) Two types of information processing in cercal systems of insects: directional sensitivity of giant interneurons. J Comp Physiol A 154:805–815
Sakaguchi DS, Murphey RK (1983) The equilibrium detecting system of the cricket. Physiology and morphology of an identified interneuron. J Comp Physiol 150:141–152
Schmidt K, Gnatzy W (1972) Die Feinstruktur der Sinneshaare auf den Cerci vonGryllus bimaculatus Deg. (Saltatoria, Gryllidae). III. Die kurzen Borstenhaare. Z Zellforsch 126:206–222
Seabrook WD (1968) The innervation of the terminal abdominal segments (VIII–XI) of the desert locust,Schistocerca gregaria. Can Entomol 100:693–715
Seabrook WD (1970) The structure of the terminal ganglionic mass of the locust,Schistocerca gregaria (Forskål). J Comp Neurol 138:63–86
Seabrook WD (1971) An electrophysiological study of the giant fiber system of the locustSchistocerca gregaria. Can J Zool 49:555–560
Shankland M (1981) Development of a sensory afferent projection in the grasshopper embryo. II. Growth and branching of peripheral sensory axons within the central nervous system. J Embryol Exp Morphol 64:187–209
Shankland M, Bentley D (1983) Sensory receptor differentiation and axonal pathfinding in the cercus of the grasshopper embryo. Dev Biol 97:468–482
Shankland M, Goodman CS (1982) Development of the dendritic branching pattern of the medial giant interneuron in the grasshopper embryo. Dev Biol 92:489–506
Shen J-X (1983) The cercus-to-giant interneuron system in the bushcricketTettigonia cantons: morphology and response to low-frequency sound. J Comp Physiol 151:449–459
Shepherd D, Kämper G, Murphey RK (1988) The synaptic origins of receptive field properties in the cricket cercal sensory system. J Comp Physiol A 162:1–11
Shimozawa T, Kanou M (1984a) Varieties of filiform hairs: range fractionation by sensory afferents and cercal interneurons of a cricket. J Comp Physiol A 155:485–493
Shimozawa T, Kanou M (1984b) The aerodynamics and sensory physiology of range fractionation in the cercal filiform sensilla of the cricketGryllus bimaculatus. J Comp Physiol A 155:495–505
Sihler H (1924) Die Sinnesorgane an den Cerci der Insekten. Zool Jb Anat 45:519–580
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Thomas JG (1965) The abdomen of the female desert locust (Schistocerca gregaria Forskål) with special reference to the sense organs. Anti-Locust Bull 42:1–20
Tyrer NM, Bell EM (1974) The intensification of profiles of cobalt-filled neurons in sectioned material. Brain Res 73:151–155
Tyrer NM, Gregory GE (1982) A guide to the neuroanatomy of locust subesophageal and thoracic ganglia. Phil Trans R Soc Lond B 297:91–124
Watson AHD, Pflüger H-J (1987) The distribution of GABA-like immunoreactivity in relation to ganglion structure in the abdominal nerve cord of the locust (Schistocerca gregaria). Cell Tissue Res 249:391–402
Westin J (1979) Responses to wind recorded from the cercal nerve of the cockroachPeriplaneta americana. I. Response properties of single sensory neurons. J Comp Physiol 133:97–102
Wine JJ (1984) The structural basis of an innate behavioural pattern. J Exp Biol 112:283–319
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boyan, G.S., Williams, J.L.D. & Ball, E.E. The wind-sensitive cercal receptor/giant interneurone system of the locust,Locusta migratoria . J. Comp. Physiol. 165, 495–510 (1989). https://doi.org/10.1007/BF00611237
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00611237