Abstract
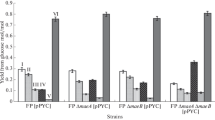
Candida maltosa JCM1504 can grow well onl-alanine as a sole carbon and nitrogen source. We found that the activities of alanine aminotransferase (AlaAT) and NAD-dependent glutamate dehydrogenase were remarkably induced when glucose-grown cells were transferred to medium containingl-alanine. This suggested thatC. maltosa has an induciblel-alanine degradation system including the above two enzymes. To assess whether AlaAT is essential for the first step ofl-alanine degradation, we isolated mutant N-07, which was unable to usel-alanine as a nitrogen source, from the wild strain. Mutant N-07 was very similar to the wild strain in terms of growth on pyruvate and on various amino acids other thanl-alanine, suggesting that N-07 lacked onlyl-alanine-assimilating ability. The AlaAT activity in the cell extract of N-07 was very low and was not induced byl-alanine, whereas the NAD-dependent glutamate dehydrogenase activity was the same as that of the wild strain and was inducible. Western blots with antibody raised against purified AlaAT fromC. maltosa indicated that no AlaAT protein was expressed in the mutant N-07. The low level of AlaAT activity described above was possibly due to the pyruvate-forming activity of other enzymes under the assay conditions. From these results, we concluded that AlaAT is an indispensable key enzyme forl-alanine assimilation inC. maltosa.
Similar content being viewed by others
References
Becher D, Schulze S, Kasuske A, Schulze H, Samsonova IA, Oliver SG (1994) Moleculer analysis of aleu2− mutant ofCandida maltosa demonstrates the presence of multiple alleles. Curr Genet 26:208–216
Bergmeyer HU, Grassl M, Walter HE (1983) Glutamate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2, 3rd edn. Academic Press, New York, pp 208–209
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang MC, Jung HK, Suzuki T, Takagi M, Yano K (1984) Ploidy in the asporogenous yeastCandida maltosa, isolation of its auxotrophic mutants and their cell fusion. J Gen Appl Microbiol 30:489–497
Cregg JM, Barringer KJ, Hessler AY, Madden KR (1985)Pichia pastoris as a host system for transformations. Mol Cell Biol 5:3376–3385
Delforge J, Messenguy F, Wiame JM (1975) The regulation of arginine biosynthesis inSaccharomyces cerevisiae. Eur J Biochem 57:231–239
Duerre JA, Chakrbarty S (1975)l-Amino acid oxidases ofProteus rettgeri. J Bacteriol 121:656–663
Hedrick LR, Dupont PD (1968) The utilization ofl-amino acids as carbon source by yeasts of the generaHansenula andTrichosporon. Antonie van Leeuwenhoek 34:465–473
Holzer H (1976) Catabolite inactivation in yeast. Trends Biochem Sci 1:178–181
Klinner U, Samsonova IA, Böttcher F (1984) Genetic analysis of the yeastCandida maltosa by means of induced parasexual processes. Curr Genet 11:241–246
Ohashima T, Soda K (1979) Purification and properties of alanine dehydrogenase fromBacillus sphaericus. Eur J Biochem 100:29–39
Richardson KE, Thompson JS (1970)l-Alanine:glyoxylate amino-transferase. Methods Enzymol, 17A:163–166
Switzer RL (1977) The inactivation of microbial enzymes in vivo. Annu Rev Microbiol 31:135–157
Umemura I, Yanagiya K, Komatsubara S, Sato T, Tosa T (1992)d-Alanine production fromdl-alanine byCandida maltosa with asymmetric degrading activity. Appl Microbiol Biotechnol 36:722–726
Umemura I, Yanagiya K, Komatsubara S, Sato T, Tosa T (1994) Purification and some properties of alanine aminotransferase fromCandida maltosa. Biosci Biotechnol Biochem 58:283–287
Yano K, Kawamura M, Takagi M (1981) Enrichment ofn-alkane assimilation-deficient mutants ofCandida yeast by synergistic effect of nystatin and pyrrolnitrin. Agric Biol Chem 45:1017–1018
Zwart KB, Overmars EH, Harder W (1983) The role of peroxisomes in the metabolism ofd-alanine in the yeastCandida utilis. FEMS Microbiol Lett 19:225–231
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Umemura, I., Yanagiya, K., Komatsubara, S. et al. ACandida maltosa mutant defective in alanine aminotransferase: isolation andl-alanine assimilation. Appl Microbiol Biotechnol 45, 519–524 (1996). https://doi.org/10.1007/BF00578465
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00578465