Abstract
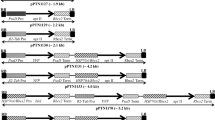
An efficient transformation system for the fungusTrichoderma longibrachiatum has been developed. Transformation was obtained both by electroporation and polyethyleneglycol treatment, using a plasmid carrying theEscherichia coli hygromycin B phosphotransferase gene as a dominant selectable marker. The transformation frequency was 0.5 to 5 transformants /μg plasmid DNA. Transformation normally occurred by tandem integration of the transforming DNA. A high percentage of the transformants were mitotically unstable. The efficiency of co-transformation was very high (around 90%), and several co-transformants containing multiple copies of theegll gene encoding a β-(1,4)-endoglucanase were obtained. Some of them secrete increased levels of endoglucanase to the culture medium. In addition, theE. coli lacZ gene was expressed in an active form under control of theAspergillus nidulans gpdA gene promoter.
Similar content being viewed by others
References
Arsdell JN van, Kwok S, Schweickart VL, Ladner M, Gelfland DH, Innis MA (1987) Cloning, characterization, and expression inSaccharomyces cerevisiae of endoglucanase I fromTrichoderma reesei. Bio/Technology 5: 60–64
Barnett CC, Berka RM, Fowler T (1991) Cloning and amplification of the gene encoding an extracellular β-glucosidase fromTrichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Bio/Technology 9: 562–567
Bergès T, Barreau C (1991) Isolation of uridine auxotrophs fromTrichoderma reesei and efficient transformation with the clonedura3 andura5 genes. Curr Genet 19: 359–365
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Chen MC, Gritzali M, Stafford WD (1987) Nucleotide sequence and deduced primary structure of cellobiohydrolase II fromTrichoderma reesei. Bio/Technology 5: 274–278
Cheng C, Tsukagoshi N, Udaka S (1990) Transformation ofTrichoderma viride using theNeurospora crassa pyr4 gene and its use in the expression of a Taka-amylase A gene fromAspergillus oryzae. Curr Genet 18: 453–456
Durand H, Baron M, Calmels T, Tiraby G (1988) Classical and molecular genetics applied toTrichoderma reesei for the selection of improved cellulolytic industrial strains. In: Paubert JP, Beguin P, Millet J (eds) Biochemistry and genetics of cellulose degradation. Academic Press, London, pp 135–151
Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction fragments to high specific activity. Anal Biochem 132: 6–13
Goldman GH, Montagu M van, Herrera-Estrella A (1990) Transformation ofTrichoderma harzianum by high-voltage electric pulse. Curr Genet 17: 169–174
González R, Ferrer S, Buesa J, Ramón D (1989) Transformation of the dermatophyteTrichophyton mentagrophytes to hygromycin B resistance. Infect Immun 57: 2923–2925
González R, Ramón D, Pérez-González JA (1992) Cloning, sequence analysis and yeast expression of theegll gene fromTrichoderma longibrachiatum. Appl Microbiol Biotechnol 38: 370–375
Gorcom RFM, Pouwels PH, Goosen T, Visser J, Broek HWJ van der, Hamer JE, Timberlake WE, Hondel CAMJJ van den (1985) Expression of anEscherichia coli β-galactosidase fusion gene inAspergillus nidulans. Gene 40: 99–106
Gruber F, Visser J, Kubicek CP, Graaf L de (1990a) The development of a heterologous transformation system for the cellulolytic fungusTrichoderma reesei based on apyrG-negative mutant strain. Curr Genet 18: 71–76
Gruber F, Visser J, Kubicek CP, Graaf L de (1990b) Cloning of theTrichoderma reesei pyrG gene and its use as a homologous marker for a high-frequency transformation system. Curr Genet 18: 447–451
Hanahan D (1983) Studies on transformation ofEscherichia coli with plasmids. J Mol Biol 166: 557–580
Herrera-Estrella A, Goldman GH, Montagu M van (1990) High-efficiency transformation system for the biocontrol agents,Trichoderma spp. Mol Microbiol 4: 839–843
Knowles JKC, Lehtovaara P, Teeri T, Penttillä ME, Salovouri T, André L (1987) The application of recombinant DNA technology to cellulases and lignocellulosic wastes. Philos Trans R Soc London [Biol] 321: 449–454
Mandels M (1985) Application of cellulases. Biochem Soc Trans 13: 414–416
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor, New York
Montenecourt BS (1983)Trichoderma reesei cellulases. Trends Biotechnol 1: 156–161
Myers AM, Tzagoloff A, Kinney DM, Lusty CJ (1986) Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction oflacZ fusions. Gene 45: 299–310
Papavizas GC (1985)Trichoderma andGliocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol 23: 23–54
Penttillä ME, Lehtovaara P, Nevalainen H, Bhikhabhai R, Knowles JKC (1986) Homology between cellulase genes ofTrichoderma reesei: complete nucleotide sequence of the endoglucanase I gene. Gene 45: 253–263
Penttillä ME, André L, Saloheimo M, Lehtovaara P, Knowles JKC (1987a) Expression of twoTrichoderma reesei endoglucanases in the yeastSaccharomyces cerevisiae. Yeast 3: 175–185
Penttillä ME, Nevalainen H, Rattö M, Salminen E, Knowles JKC (1987b) A versatile transformation system for the cellulolytic filamentous fungusTrichoderma reesei. Gene 61: 155–164
Pérez-González JA, González R, Querol A, Sendra J, Ramón D (1993) Construction of a recombinant wine yeast strain expressing a β-(1,4)-endoglucanase activity and its use in microvinification experiments. Appl Environ Microbiol 59: 2801–2806
Pontecorvo G, Ropper JA, Jemmons LJ, MacDonald KD, Buft AWJ (1953) The genetics ofAspergillus nidulans. Adv Genet 5: 141–238
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, Hondel CAMJJ van den (1987) Transformation ofAspergillus based on the hygromycin B resistance marker fromEscherichia coli. Gene 56: 117–124
Ruiz-Sala P, Pérez-González JA, Ramón D (1993) Nucleotide sequence of aTrichoderma longibrachiatum DNA fragment encoding the 5.8S rRNA gene. Nucleic Acids Res 21: 741
Saloheimo M, Lehtovaara P, Penttillä ME, Teeri TT, Stahlberg J, Petterson G, Claeyssens M, Tomme P, Knowles JK (1988) EGIII, a new endoglucanase fromTrichoderma reesei: the characterization of both gene and enzyme. Gene 63: 11–21
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning:a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
Shoemaker S, Schweickart V, Ladner M, Gelfand D, Kowk S, Myambo K, Innis M (1983) Molecular cloning of exo-cellobiohydrolase I derived fromTrichoderma reesei strain L27. Bio/technology 1: 691–696
Sivan A, Stasz TE, Hemmat M, Hayes CK, Harman GE (1992) Transformation ofTrichoderma spp. with plasmids conferring hygromycin B resistance. Mycologia 84: 687–694
Smith JL, Bayliss FT, Ward M (1991) Sequence of the clonedpyr4 gene ofTrichoderma reesei and its use as homologous selectable marker for transformation. Curr Genet 19: 27–33
Sternberg D (1976) Production of cellulase byTrichoderma. Biotechnol Bioeng Symp 6: 35–53
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes inTrichoderma reesei by sophorose. J Bacteriol 139: 761–769
Teeri TT, Lehtovaara P, Kauppinen S, Salovouri I, Knowles JKC (1987) Homologous domains inTrichoderma reesei cellulolytic enzymes:gene sequence and expression of cellobiohydrolase II. Gene 51: 43–52
Ulhoa CJ, Vainstein MH, Peberdy JF (1992) Transformation ofTrichoderma species with dominant selectable markers. Curr Genet 21: 23–26
Ventura L, Ramón D (1991) Transformation ofAspergillus terreus with the hygromycin B resistance marker fromEscherichia coli. FEMS Microbiol Lett 82: 189–194
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160: 87–112
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sánchez-Torres, P., González, R., Pérez-González, J.A. et al. Development of a transformation system forTrichoderma longibrachiatum and its use for constructing multicopy transformants for theegl1 gene. Appl Microbiol Biotechnol 41, 440–446 (1994). https://doi.org/10.1007/BF00939033
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00939033