Abstract
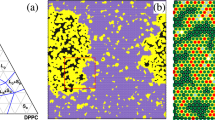
The softening of wet lipid bilayer membranes during their gel-to-fluid first-order phase transition is studied by computer simulation of a family of two-dimensional microscopic interaction models. The models include a variable number, q, of lipid chain conformational states, where 2≦q≦10. Results are presented as functions of q and temperature for a number of bulk properties, such as internal energy, specific heat, and lateral compressibility. A quantitative account is given of the statistics of the lipid clusters which are found to form in the neighborhood of the transition. The occurrence of these clusters is related to the softening and the strong thermal density fluctuations which dominate the specific heat and the lateral compressibility for the high-q models. The cluster distributions and the fluctuations behave in a manner reminiscent of critical phenomena and percolation. The findings of long-lived metastable states and extremely slow relaxational behavior in the transition region are shown to be caused by the presence of intermediate lipid chain conformational states which kinetically stabilize the cluster distribution and the effective phase coexistence. This has as its macroscopic consequence that the first-order transition apperas as a “continuous” transition, as invariably observed in all experiments on uncharged lecithin bilayer membranes. The results also suggest an explanation of the non-horizontal isotherms of lipid monolayers. Possible implications of lipid bilayer softening and enhanced passive permeability for the functioning of biological membranes are discussed.
Similar content being viewed by others
Abbreviations
- PC:
-
phosphatidvlcholine
- DMPC:
-
dimyristoyl PC
- DPPC:
-
dipalmitoyl PC
- ac:
-
alternating current
- DSC:
-
differential scanning calorimetry
- T m :
-
lipid gel-to-fluid phase transition temperature
- TEMPO:
-
2,2,6,6-tetramethylpiperidine-N-oxyl
References
Albon N, Sturtevant JM (1978) Nature of the gel to liquid crystal transition of synthetic phosphatidylcholines. Proc Natl Acad Sci USA 75:2258–2260
Albrecht O, Gruler H, Sackmann E (1978) Polymorphism of phospholipid monolayers. J Phys (Paris) 39:301–313
Black SG, Dixon GS (1981) Ac calorimetry of dimyristoylphosphatidylcholine multilayers: Hysteresis and annealing near the gel to liquid-crystal transition. Biochemistry 20: 6740–6744
Cadenhead DA, Müller-Landau F, Kellner BMJ (1980) Phase transition in insoluble one and two-component films at the air/water interface. In: Sinha K (ed) Ordering in two dimensions. North-Holland, New York, pp 73–81
Caillé A, Pink DA, de Verteuil F, Zuckermann MJ (1980) Theoretical models for quasi-two-dimensional mesomorphic monolayers and membrane bilayers. Can J Phys 58:581–611
Carruthers A, Melchior DL (1983) Studies of the relationship between water permeability and bilayer physical state. Biochemistry 22:5797–5807
Davis JM (1979) Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J 27:339–358
de Gier J, Mandersloot JG, van Deenen LLM (1968) Lipid composition and permeability of liposomes. Biochim Biophys Acta 150:666–675
Doniach S (1978) Thermodynamic fluctuations in phospholipid bilayers. J Chem Phys 68:4912–4916
Evans E, Kwok R (1982) Mechanical calorimetry of large dimyristoylphosphatidylcholine vesicles in the phase transition region. Biochemistry 21:4874–4879
Fischer A, Sackmann E (1984) Electron microscopy and diffraction study of phospholipid monolayers transferred from water to solid substrates. J Phys (Paris) 45:517–527
Fischer A, Lösche M, Möhwald H, Sackmann E (1984) On the nature of the lipid monolayer phase transition. J Phys Lett (Paris) 45:L785-L791
Freire E, Biltonen R (1978) Estimation of molecular averages and equilibrium fluctuations in lipid bilayer systems from excess heat capacity function. Biochim Biophys Acta 514: 54–68
Gebhardt C, Gruler H, Sackmann E (1977) On domain structure and local curvature in lipid bilayers and biological membranes. Z. Naturforsch 32c:581–596
Georgallas A, Hunter DL, Lookman T, Zuckermann MJ, Pink DA (1984) Interaction between two sheets of a bilayer membrane and its internal lateral pressure. Eur Biophys J 11:79–86
Georgallas A, Pink DA (1982) A new theory of the liquid condensed-liquid expanded phase transition in lipid monolayers. Can J Phys 60:1678–1681
Hatta I, Suzuki K, Imaizumi S (1983) Pseudo-critical heat capacity of single lipid bilayers. J Phys Soc Jpn 52: 2790–2797
Hatta I, Imaizumi S, Akutsu Y (1984) Evidence for weak first-order nature of lipid bilayer phase transition from the analysis of pseudo-critical specific heat. J Phys Soc Jpn 53:882–888
Hui SW, Parsons DF (1975) Direct observation of domains in wet lipid bilayers. Science 190:383–384
Israelachvili JN, Marcelja S, Horn RG (1981) Physical principles of membrane organization. Q Rev Biophys 13: 121–200
Jänig F (1981) Critical effects from lipid-protein interaction in membranes. II. Interpretation of experimental results. Biophys J 36:347–357
Kanehisa MI, Tsong TY (1978) Cluster model of lipid phase transitions with application to passive permeation of molecules and structure relaxation in lipid bilayers. J Am Chem Soc 100:424–432
Lee AG (1977) Lipid phase transitions and phase diagrams I. Lipid phase transitions. Biochim Biophys Acta 472: 237–281
Lis LJ, McAlister M, Fuller N, Rand RP, Parsegian VA (1982) Measurements of the lateral compressibility of several phospholipid bilayers. Biophys J 37:667–672
Mabrey S, Sturtevant JM (1976) Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc Natl Acad Sci USA 73:3862–3866
Marcelja S (1974) Chain ordering in liquid crystals II. Structure of bilayer membranes. Biochim Biophys Acta 367: 165–176
Marcelja S, Wolfe J (1979) Properties of bilayer membranes in the phase transition or phase separation region. Biochim Biophys Acta 557:24–31
Marsh D, Watts A, Knowles PF (1976) Evidence for phase boundary lipid. Permeability of Tempo-choline into dimyristoylphosphatidylcholine vesicles at the phase transition. Biochemistry 15:3570–3578
McCammon JA, Deutch JM (1975) Semiempirical models for biomembrane phase transitions and phase separations. J Am Chem Soc 97:6675–6681
McElhaney RN (1982) Effects of membrane lipids on transport and enzymatic activities. Curr Topics Membr Transport 17: 317–380
McElhaney RN (1984) The structure and function of the Acholeplasma Laidlawii plasma membrane. Biochim Biophys Acta 779:1–42
McKay AL (1981) A proton NMR moment study of the gel and liquid-crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J 35:301–313
Mitaku S, Ikegami A, Sakanishi A (1978) Ultrasonic studies of lipid bilayer. Phase transition in synthetic phosphatidylcholine liposomes. Biophys Chem 8:295–304
Mitaku S, Jippo T, Kataoka R (1983) Thermodynamic properties of the lipid bilayer transition. Pseudocritical behavior. Biophys J 42:137–144
Mouritsen OG (1983) Studies on the lack of cooperativity in the melting of lipid bilayers. Biochim Biophys Acta 731: 217–221
Mouritsen OG (1984) Computer studies of phase transitions and critical phenomena. Springer, Berlin Heidelberg New York
Mouritsen OG, Boothroyd D, Harris R, Jan N, Lookman T, MacDonald L, Pink DA, Zuckermann MJ (1983) Computer simulation of the main gel-fluid phase transition of lipid bilayers. J Chem Phys 79:2027–2041
Nagle JF (1980) Theory of the main lipid bilayer phase transition. Annu Rev Phys Chem 31:157–195
Nagle JF, Scott HL (1978) Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta 513:236–243
Papahadjopoulos D, Jacobsen K, Nir S, Isac T (1973) Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta 311: 330–348
Quinn PJ, Chapman D (1980) The dynamics of membrane structure. CRC Crit Rev Biochem 8:1–117
Rüppel D, Sackmann E (1983) On defects in different phases of two-dimensional lipid bilayers. J Phys (Paris) 44: 1025–1034
Sackmann E, Rüppel D, Gebhardt C (1980) Defect structure and texture of isolated bilayers of phospholipids and phospholipid mixtures. In: Helfrich W, Heppke G (eds) Liquid crystals of one-and two-dimensional order. Springer, Berlin Heidelberg New York (Springer Series in Chemical Physics, vol 11, pp 309–326)
Sandermann H (1978) Regulation of membrane enzymes by lipids. Biochim Biophys Acta 515:209–237
Stanley HE (1971) Introduction to phase transitions and critical phenomena. Clarendon Press, Oxford
Stauffer D (1979) Scaling theory of percolating clusters. Phys Rep 54:1–74
Stoll E, Binder K, Schneider T (1972) Evidence for Fisher's droplet model in simulated two-dimensional cluster distributions. Phys Rev B 6:2777–2780
Träuble H (1971) The movement of molecules across lipid membranes: A molecular theory. J Membr Biol 4:193–208
Tsong TY, Geenberg M, Kanehisa MI (1977) Anesthetic action on membrane lipids. Biochemistry 16:3115–3121
von Tscharner V, McConnell HM (1981) An alternative view of phospholipid phase behavior at the air-water interface. Biophys J 36:409–419
Zuckermann MJ, Pink DA (1980) The correlation length and lateral compressibility of phospholipid bilayers in the presence of thermodynamic density fluctuations. J Chem Phys 73:2919–2926
Zuckermann MJ, Pink DA, Costas M, Sanctuary BC (1982) A theoretical model for phase transitions in lipid monolayers. J Chem Phys 76:4206–4216
Author information
Authors and Affiliations
Additional information
Supported by the Danish Natural Science Research Council and A/S De Danske Spritfabrikkers Jubilæumslegat
Supported in part by the NSERC of Canada and Le FCAC du Quebec
Rights and permissions
About this article
Cite this article
Mouritsen, O.G., Zuckermann, M.J. Softening of lipid bilayers. Eur Biophys J 12, 75–86 (1985). https://doi.org/10.1007/BF00260430
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00260430