Summary
This study examined the effect of metyrapone on the elimination rate of acetaminophen and on the apparent formation rate of acetaminophen metabolites in man.
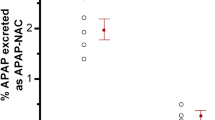
Metyrapone treatment, 1.5 g, increased the half-life of acetaminophen, decreased the fraction of the dose recovered in the urine as the glucuronide and increased the fraction of the dose recovered in urine as the sulfate and mercapturate conjugates. The apparent rate constant for the formation of acetaminophen glucuronide was significantly decreased by metyrapone while the apparent rate constants for the formation of the sulfate and mercapturic acid metabolites were unchanged or slightly increased, respectively.
These data indicate that metyrapone inhibits acetaminophen glucuronidation and possibly enhances the oxidation of acetaminophen to its quantitatively minor yet highly toxic reactive metabolite. The extent to which the parallel pathways of acetaminophen elimination are also affected by inhibitors of cytochrome P-450-mediated oxidation will limit the efficacy of these types of potential antidotes for the treatment of acetaminophen overdose.
Similar content being viewed by others
References
Prescott LF, Wright N, Roscoe P, Brown SS (1971) Plasmaparacetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet 1: 519–522
Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187: 185–194
Anon. (1985) Suicide and suicide attempts by the nonmedical use of drugs. Morbid Mortal Weekly Rep 34: 570–571
Slattery JT, Levy G (1979) Acetaminophen kinetics in acutely poisoned patients. Clin Pharmacol Ther 25: 184–195
Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. III. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther 187: 203–210
Jollow DJ, Thorgeirsson SS, Potter WZ, Hashimoto M, Mitchell JR (1974) Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology 12: 251–271
Prescott LF (1981) Treatment of severe acetaminophen poisoning with intravenous acetylcysteine. Arch Intern Med 141: 386–389
Mitchell MC, Schenker S, Avant GR, Speeg KV (1981) Cimetidine protects against acetaminophen hepatotoxicity in rats. Gastroenterology 81: 1052–1060
Abernethy DR, Greenblatt DJ, Divoll M, Ameer B, Shader RI (1983) Differential effect of cimetidine on drug oxidation (antipyrine and diazepam) vs. conjugation (acetaminophen and lorazepam): Prevention of acetaminophen toxicity by cimetidine. J Pharmacol Exp Ther 224: 508–513
Critchley JAJH, Dyson EH, Scott AW, Jarvie DR, Prescott LF (1983) Is there a place for cimetidine or ethanol in the treatment of paracetamol poisoning? Lancet 1: 1375–1376
Mitchell MC, Schenker SS and Speeg KV (1984) Selective inhibition of acetaminophen oxidation and toxicity by cimetidine and other histamine H2-receptor antagonists in vivo and in vitro in the rat and in man. J Clin Invest 73: 383–391
Miners JO, Attwood J, Birkett DJ (1984) Determinants of acetaminophen metabolism: Effect of inducers and inhibitors of drug metabolism on acetaminophen's metabolic pathways. Clin Pharmacol Ther 35: 480–486
Liebman KC (1969) Effects of metyrapone on liver microsomal drug oxidations. Mol Pharmacol 5: 1–9
Jenkins JS, Meakin JW, Nelson DH, Thorn GW (1958) Inhibition of adrenal steroid 11-oxygenation in the dog. Science 128: 478–479
Netter KJ, Jenner S, Kajuschke (1967) Über die Wirkung von Metyrapon auf den Mikrosomalen Arzneimittelabbau. Naunyn Schmiedebergs Arch Pharmacol 259: 1–16
Goldstein M, Nelson EB (1979) Metyrapone as a treatment for acetaminophen (paracetamol) toxicity in mice. Res Commun Chem Pathol Pharmacol 23: 203–206
Nelson EB, Montes M, Goldstein M (1980) Effectiveness of metyrapone in the treatment of acetaminophen toxicity in mice. Toxicology 17: 73–81
Corcoran GB, Todd EL, Racz WJ, Hughes H, Smith CV, Mitchell JR (1985) Effect of N-acetylcysteine on the disposition and metabolism of acetaminophen in mice. J Pharmacol Exp Ther 232: 857–863
Miners J, Adams JF, Birkett DJ (1984) A simple HPLC assay for urinary paracetamol metabolites and its use to characterize the C3H mouse as a model for paracetamol metabolism studies. Clin Exp Pharmacol Physiol 11: 209–217
Ray K, Adithan C, Bapna JS, Kangle PR, Ramakrishnan S (1985) Effect of short surgical procedures on salivary paracetamol elimination. Br J Clin Pharmacol 20: 174–176
Lowenthal DT, Øie S, Van Stone JC, Briggs WA, Levy G (1976) Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther 196: 570–578
Kamali F, Fry JR, Bell GD (1987) Salivary secretion of paracetamol in man. J Pharm Pharmacol 39: 150–152
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York, NY
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice Hall, Englewood Cliffs, New Jersey, pp 122–149
Adithan C, Thangam J (1982) A comparative study of saliva and serum paracetamol levels using a simple spectrophotometric method. Br J Clin Pharmacol 14: 107–109
Prescott LF (1980) Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 10: 291S-298S
Galinsky RE, Levy G (1984) Evaluation of activated charcoal-sodium sulfate combination for inhibition of acetaminophen absorption and repletion of inorganic sulfate. Clin Toxicol 22: 21–30
Slattery JT, Wilson JM, Kalhorn TF, Nelson SD (1987) Dose-dependent pharmacokinetics of acetaminophen: Evidence of glutathione depletion in humans. Clin Pharmacol Ther 41: 413–418
Divoll M, Greenblatt DJ, Ameer B, Abernethy DR (1981) Effect of food on acetaminophen absorption in young and elderly subjects. J Clin Pharmacol 22: 571–576
Cinti DL (1982) Agents activating the liver microsomal mixed function oxidase system. In: Schenkman J, Kupfer D (eds) Hepatic cytochrome P-450 monooxygenase system. Pergamon Press, New York, NY
Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H (1974) Acetaminophen-induced hepatic injury: Protective role of glutatione in man and rationale for therapy. Clin Pharmacol Ther 16: 676–684
Rollins DE, Glaumann H, Moldéus P, von Bahr C (1979) Acetaminophen conjugation by human adult liver. Fed Proc (abstract) 38: 680
Kahl GF (1970) Experiments on the metyrapone reducing microsomal enzyme system. Naunyn Schmiedebergs Arch Pharmacol 266: 61–74
Maser E, Legrum W (1985) Alteration of the inhibitory effect of metyrapone by reduction to metyrapol during the metabolism of methacetin in vivo in mice. Naunyn Schmiedebergs Arch Pharmacol 331: 283–289
Hannah DM, Sprunt JG (1969) The quantitation of metyrapone and its reduced derivative in urine. J Pharm Pharmacol 21: 877–878
Crooks PA, Damani LA, Cowan DA (1981) Synthesis of N-oxide derivatives of metyrapone and their detection as in vitro metabolites. J Pharm Pharmacol 33: 309–312
Hjelle JJ, Hazelton GA, Klaassen CD (1985) Acetaminophen decreases adenosine 3′-phosphate 5′-phosphosulfate and uridine diphosphoglucuronic acid in rat liver. Drug Metab Dispos 13: 35–41
Howell SR, Hazelton GA, Klaassen CD (1986) Depletion of UDP-glucuronic acid by drugs that are glucuronidated. J Pharmacol Exp Ther 236: 610–614
Abernethy DR, Greenblatt DJ, Ameer B, Shader RI (1985) Probenecid impairment of acetaminophen and lorazepam clearance: Direct inhibition of ether glucuronide formation. J Pharmacol Exp Ther 234: 345–349
Jollow DJ, Smith C (1977) Biochemical aspects of toxic metabolites: Formation, detoxication, and covalent binding. In: Jollow DJ, Kocsis JJ, Snyder R, Vainio H (eds) Biological reactive intermediates. Formation, toxicity and inactivation. Plenum Press, New York, NY
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Galinsky, R.E., Nelson, E.B. & Rollins, D.E. Pharmacokinetic consequences and toxicologic implications of metyrapone-induced alterations of acetaminophen elimination in man. Eur J Clin Pharmacol 33, 391–396 (1987). https://doi.org/10.1007/BF00637636
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00637636