Abstract
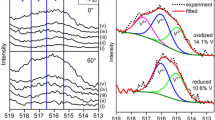
The formation of vanadium surface oxides and their influence on the absorption of hydrogen were studied by XPS and TDMS methods. Vanadium hereby serves as a model system for hydrogen metal interaction. Different stable and unstable oxides indicated by the oxidation number of vanadium have been investigated: oxidation number +5 corresponding to stable V2O5; oxidation number +3 corresponding to stable V2O3 and lower than +3, corresponding to unstable oxides. Exposure of a cleaned V sample to different oxygen dosages (1 L – 1000 L, pO2 = 1 × 10–4 Pa) at room temperature leads to the formation of unstable oxides with oxidation numbers smaller than 3. V-O bondings break up at temperatures higher than 290 °C and oxygen desorbs. Besides the dosage the oxide formation is also influenced by the temperature. All these oxides act as surface barriers and prevent the absorption of hydrogen by vanadium.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 2 November 1998 / Revised: 22 March 1999 / Accepted: 24 March 1999
Rights and permissions
About this article
Cite this article
Kiss, G., Paulus, H., Krafcsik, O. et al. Effect of surface oxidation on the solution of hydrogen in vanadium. Fresenius J Anal Chem 365, 203–207 (1999). https://doi.org/10.1007/s002160051473
Issue Date:
DOI: https://doi.org/10.1007/s002160051473