Abstract
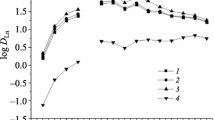
The extraction behavior of tervalent rare-earth metals (Ln) using a heptane solution containing bis(2-ethylhexyl)phosphinic acid (PIA-8, HR) from 0.1 mol/dm3 sodium perchlorate media was studied. The pH0.5 values and separation factors obtained were compared among the metals. The stoichiometry of the extracted species and the extraction constants for the present aqueous/heptane system were determined by slope analysis. It is demonstrated that the rare-earth metals were extracted as monomers LnR3⋅mHR (m=3, 4, 5 or 6), and the extracted species could be stripped into a relatively low concentrated hydrochloric acid. PIA-8 was found to be the most selective extractant for the mutual separation of rare-earth metals among the other phosphinic acids reported.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 February 1996/Revised: 13 May 1996/Accepted: 21 May 1996
Rights and permissions
About this article
Cite this article
Nagaosa, Y., Binghua, Y. Solvent extraction of rare-earth metals with bis(2-ethylhexyl)phosphinic acid. Fresenius J Anal Chem 357, 635–641 (1997). https://doi.org/10.1007/s002160050226
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002160050226