Abstract
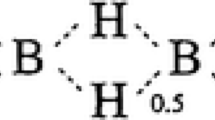
The energies (i.e. energy expectation values) and weights of various N2O4 valency structures are reported. Certain structures that apparently violate the octet rule are of low energy. This is because their wave functions allow for considerable resonance interaction between orthogonal wave functions that represent non-octet violating structures.
Zusammenfassung
Erwartungswerte der Energien und Gewichte verschiedener Valenz-Strukturen des N2O4 werden mitgeteilt. Einige scheinbar die Oktett-Regel verletzende Strukturen sind energiearm: es sind Hybridstrukturen nicht die Oktett-Regel verletzender Valenzstrukturen.
Résumé
Les énergies (espérances mathématiques de l'énergie) et les poids de diverses structures de valence de N2O4 sont donnés. Certaines structures qui violent apparemment la regle de l'octet ont une basse énergie. Ceci est dû à ce que leurs fonctions d'ondes permettent une forte interaction de résonance entre fonctions d'onde orthogonales qui représentent des structures respectant la regle de l'octet.
Similar content being viewed by others
References
Brown, R. D., and R. D. Harcourt: Proc. chem. Soc. (London) 1961, 216.
— —: Austral. J. Chem. 16, 737 (1963).
— —: Austral. J. Chem. 18, 1115 (1965).
Gould, R. D., and J. W. Linnett: Trans. Faraday Soc. 59, 1001 (1963).
Harcourt, R. D.: Theoret. chim. Acta 2, 437 (1964).
—: Theoret. chim. Acta 3, 194, 290 (1965).
—: Theoret. chim. Acta 4, 202 (1966).
Hirst, D. M., and J. W. Linnett: J. chem. Soc. (London) 1962, 1035.
Linnett, J. W.: The electronic structures of molecules, p. 61. London: Methuen 1964.
—, and R. M. Rosenberg: Tetrahedron 20, 53 (1964).
McEwen, K. L.: J. chem. Physics 32, 1801 (1960).
Murrell, J. N., and K. L. McEwen: J. chem. Physics 25, 1143 (1956).
—, and D. R. Williams: Proc. Roy. Soc. (London) A 284, 566 (1965).
Pauling, L.: Nature of the chemical bond, p. 185. New York: Cornell Univ. Press 1960.
—: Nature of the chemical bond, p. 187. New York: Cornell Univ. Press 1960.
—: Nature of the chemical bond, p. 350. New York: Cornell Univ. Press 1960.
Peters, D.: J. amer. chem. Soc. 84, 3812 (1962).
Samuel, R.: J. chem. Physics 12, 167, 180, 380, 521 (1944).
—: J. chem. Physics 13, 572 (1945).
Takekiyo, S.: Bull. chem. Soc. Japan 34, 1466 (1961).
Wheland, G. W.: J. chem. Physics 13, 239 (1945).
Author information
Authors and Affiliations
Additional information
I desire to express my thanks to Dr. C. G. Barraclough for reading the manuscript.
Rights and permissions
About this article
Cite this article
Harcourt, R.D. Valency structures for N2O4 part II. Theoret. Chim. Acta 6, 131–140 (1966). https://doi.org/10.1007/BF00526944
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00526944