Abstract
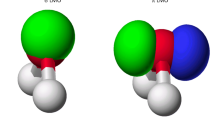
Localized orbitals are derived for the ground states of the oxygen and nitric oxide molecules by applying localization methods separately to the orbitals containing electrons of alpha- and betaspin. Both the intrinsic energy localization and the uniform localization methods are used. The resulting localized orbitals are in good agreement with the formulae suggested by Linnett.
Zusammenfassung
Für die Grundzustände der Moleküle O2 und NO werden lokalisierte Orbitale abgeleitet, indem die Lokalisierungsmethoden getrennt für die Orbitale mitα- undβ-Spin angewendet werden. Dabei werden sowohl die eigentlichen Energielokalisierungsmethoden als auch die gleichförmigen Lokalisierungsmethoden benutzt. Die erhaltenen lokalisierten Orbitale stimmen gut mit den von Linnett vorgeschlagenen Formeln überein.
Résumé
Obtention d'orbitales localisées pour l'état fondamental des molécules d'oxygène et d'oxyde nitreux par localisation indépendante des orbitales de spinα et des orbitales de spinβ. Emploi simultané de la localisation selon l'énergie intrinsèque et de la localisation uniforme. Les orbitales localisées résultantes sont en bon accord avec les formules suggérées par Linnett.
Similar content being viewed by others
References
Edmiston, C., Ruedenberg, K.: Rev. mod. Physics35, 457 (1963); - J. chem. Physics43, S 97 (1965);- Quantum theory of atoms, molecules and the solid state, p. 263. Ed. Löwdin, P. O. New York: Academic Press Inc. 1966.
Murrell, J. N., Stamper, J. G., Trinajstić, N.: J. chem. Soc. (London)A 1966, 1624.
Peters, D.: J. chem. Soc. (London)1963, 2003.
Magnasco, V., Perico, A.: J. chem. Physics47, 971 (1967);48, 800 (1968).
Linnett, J. W.: J. Amer. chem. Soc.83, 2643 (1961); - Electronic structure of molecules. London: Methuen 1964.
Ruedenberg, K.: Modern quantum chemistry. Part 1, p. 85, ed. Sinanoglu, O. NewYork: Academic Press Inc. 1965.
Lennard-Jones, J., Pople, J. A.: Proc. Roy. Soc. (London)A202, 166 (1950).
Sahni, R. C., Lorenzo, E. J.: J. chem. Physics42, 3612 (1965).
Hopgood, F. R. A.: MIDIAT on Atlas, a version of the diatomic integral program QCPE 29 (Quantum Chemistry Program Exchange, Indiana University, Bloomington, Indiana) of F. J. Corbato and A. C. Switendick: Methods in computational physics, Vol. II, p. 155, ed. B. Alder, S. Fernbach and M. Rotenberg. New York: Academic Press 1963.
Segal, G. A.: QCPE91 (Quantum Chemistry Program Exchange, Indiana University, Bloomington, Indiana).
Brion, H., Moser, C., Yamazaki, M.: J. chem. Physics30, 673 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirst, D.M., Linington, M.E. Localized orbitals for the oxygen and nitric oxide molecules. Theoret. Chim. Acta 16, 55–62 (1970). https://doi.org/10.1007/BF01045967
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01045967