Summary
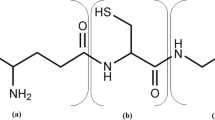
The incorporation of [35S]-sulphate was followed into washed-cell suspensions of Nitrobacter agilis. Thus, bound sulphate, sulphite, sulphide, cysteine, glutathione, homocysteine, methionine and taurine were detected in the ethanol-soluble fraction as well as in the residual hydrolysed fraction. The reaction between thiol groups and N-ethylmaleimide has been successfully used to stabilize the SH-compounds in cell extracts, and the derivatives thus obtained were separated by paper chromatography.
A soluble enzyme system catalyzing the reduction of sulphate to sulphite has been prepared. As a result of DEAE-cellulose-11 column chromatography, the enzyme complex was cleaved into two protein bands, one containing ATP-sulphurylase and the other APS-kinase and PAPS-reductase. The last two enzymes were further purified by DEAE-sephadex and Sephadex G-150 column chromatography. At pH 7.6 the enzymes show maximal activity in the presence of ATP and an ATP-generating system (creatine phosphate and creatine phosphokinase), APS, NADP+, a NADP+-reducing system (glucose-6-phosphate and a glucose-6-phosphate dehydrogenase) and MgCl2. Addition of small amounts of 2,3-dimercaptopropan-1-ol (BAL) to the buffers stabilized the enzymes and enabled them to be dialyzed for 16 h, without loss of activity. Anaerobic conditions are required for maximal activity.
The optimum concentration of various cofactors for enzyme activity has been determined. The K m values are as follows: ATP, 1.3×10-3 M; APS, 1.6×10-4 M and NADP+, 1.8×10-3 M. The molecular weight of the APS-kinase and PAPS-reductase complex is about 280000. The PCMB inhibition of the two enzymes is reversed by adding GSH, L-cysteine and Cleland's reagent.
Similar content being viewed by others
Abbreviations
- APS:
-
adenosine 5′-phosphosulphate
- PAPS:
-
3′-phosphoadenosine 5′-phosphosulphate
- PCMB:
-
p-chloromercuribenzoate
- NEM:
-
N-ethylmaleimide
- PPO:
-
2,5-diphenyloxazole
- POPOP:
-
1,4-bis-(5-phenyloxazole-2)-benzene; Cleland's reagent, dithiothreitol
References
Andrews, P.: The gel-filtration behavior of proteins related to their molecular weights over a wide range. Biochem. J. 96, 595–606 (1965).
Asahi, T.: Sulphur metabolism in higher plants. IV. Mechanism of sulphur reduction in chloroplasts. Biochim. biophys. Acta (Amst.) 82, 58–66 (1964).
—, Bandurski, R. S., Wilson, L. G.: Yeast sulphate reducing system. II. Enzymatic reduction of protein disulphide. J. biol. Chem. 236, 1831–1835 (1961).
Baddiley, J., Buchanan, J. G., Letters, R.: Synthesis of adenosine-5′-phosphosulphate. A degradation product of an intermediate in the enzymic synthesis of sulphuric esters. Pt. I, J. Chem. Soc., pp. 1067–1071 (1957).
Balharry, G. J. E., Nicholas, D. J. D.: ATP-sulphurylase in spinach leaves. Biochim. biophys. Acta (Amst.) 220, 513–524 (1970).
Determann, H.: Gel chromatography, A laboratory handbook. 2nd Edition, p. 13. Berlin: Springer 1969.
Ellis, R. J.: Sulphur metabolism: The usefulness of N-ethylamaleimide. Nature (Lond.) 211, 1266–1268 (1966).
Harris, G. K., Tigone, E., Hanes, G. S.: Quantitative chromatographic methods. 7. Isolation of amino acids from serum and other fluids. Canad. J. Biochem. Physiol. 39, 439–451 (1961).
Hodson, R. C., Schiff, J. A., Scarsella, A. J., Levinthal, M.: Studies of sulphate utilization by algae. 6. Adenosine-3′-phosphate-5′-phosphosulphate (PAPS) as an intermediate in thiosulphate formation from sulphate by cell-free extracts of Chlorella. Plant Physiol. 43, 563–569 (1968).
Horowitz, N. H.: In: A symposium on amino acid metabolism. (Eds. W. D. McElroy and H. B. Glass.) Discussion, pp. 631–632. Baltimore: John Hopkins University Press 1955.
Lampen, J. O., Roepke, R. R., Jones, M. J.: Studies on the sulphur metabolism of Escherichia coli. III. Mutant strains of Escherichia coli unable to utilize sulphate for their complete sulphur requirements. Arch. Biochem. 13, 55–66 (1947).
Layne, E.: In: S. P. Colowick and N. O. Kaplan: Methods in enzymology, vol. 3, pp. 451–456. New York-London: Academic Press 1957.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
Margolis, D., Mandle, R. N.: A system for separating sulphur and non-sulphur amino acid compounds by two dimensional paper chromatograms. Boyce Thomps. Inst. Contribut. 19, 509–512 (1958).
Naiki, N.: Enzymatic defects in sulphate reducing system of sulphite-less yeast mutants. Plant and Cell Physiol. 5, 71–78 (1964).
Porque, P. G., Baldesten, A., Reichard, P.: The involvement of the thioredoxin system in the reduction of methionine sulphoxide and sulphate. J. biol. Chem. 245, 2371–2374 (1970).
Prabhakararao, K., Nicholas, D. J. D.: Sulphite reductase from baker's yeast: A haemoflavoprotein. Biochim. biophys. Acta (Amst.) 180, 253–263 (1969).
Robbins, P. W., Lipmann, F.: Separation of the two enzymatic phases in active sulphate synthesis. J. biol. Chem. 233, 681–685 (1958).
Roberts, R. B., Abelson, P. H., Cowie, D. B., Bolton, E. T., Britton, R. J.: Sulphur metabolism. In: Studies of biosynthesis in Escherichia coli, pp. 318–405. Washington: Carnegie Inst. Publ. 1955.
Schlossmann, K., Lynen, F.: Biosynthesis of cysteine from serine and H2S. Biochem. Z. 328, 591–594 (1957).
Smith, I.: Chromatographic and electrophoretic techniques, vol. 2, p. 210, London: Heinemann 1960.
Tate, M. E.: Separation of myoinositol pentaphosphates by moving paper electrophoresis (MPE). Analyt. Biochem. 23, 141–149 (1968).
Thiele, H. H.: Sulphur metabolism in Thiorhodaceae. V. Enzymes of sulphur metabolism in Thiocapsa floridana and Chromatium species. Antonie v. Leeuwenhoeck 34, 350–356 (1968).
Torii, K., Bandurski, R. S.: Yeast sulphate-reducing system. III. An intermediate in the reduction of 3′-phosphoryl-5′-adenosine phosphosulphate to sulphite. Biochim. biophys. Acta (Amst.) 136, 286–295 (1967).
Trudinger, P. A.: Assimilatory and dissimilatory metabolism of inorganic sulphur compounds by microorganisms. In: Advances in microbial physiology, Vol. 3, pp. 111–152. Ed. by A. H. Rose and J. F. Wilkinson. New York: Academic Press 1969.
Varma, A.K., Nicholas, D.J.D.: Assimilation of sulphate by nitrifying bacteria. Proc. Aust. Biochem. Soc. p. 61 (1969).
——: Studies on the incorporation of labelled sulphate into cells and cell-free extracts of Nitrosomonas europeae. Arch. Mikrobiol. 73, 293–307 (1970).
——: Purification and properties of ATP-sulphurylase from Nitrobacter agilis. Biochim. biophys. Acta (Amst.) 227, 373–389 (1971).
Wainwright, W. W., Anderson, E. C., Hammer, P. C., Lehman, C. A.: Simplified autoradiography exposure calculation. Nucleonics 12, 19–21 (1954).
Wallace, W., Nicholas, D. J. D.: Properties of some reductase enzymes in the nitrifying bacteria and their relationship to the oxidase systems. Biochem. J. 109, 763–773 (1968).
Warburg, O.: Wasserstoff übertragende Fermente. Berlin: W. Saenger 1948.
Wilson, L. G., Asahi, T., Bandurski, R. S.: Yeast sulphate reducing system. I. Reduction of sulphate to sulphite. J. biol. Chem. 236, 1822–1829 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Varma, A.K., Nicholas, D.J.D. Metabolism of 35S-sulphate and properties of APS-kinase and PAPS-reductase in Nitrobacter agilis . Archiv. Mikrobiol. 78, 99–117 (1971). https://doi.org/10.1007/BF00424867
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00424867