Abstract
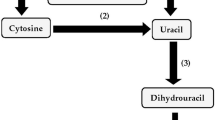
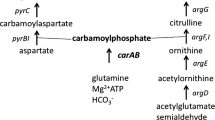
The five de novo enzyme activities unique to the pyrimidine biosynthetic pathway were found to be present in Pseudomonas pseudoalcaligenes ATCC 17440. A mutant strain with 31-fold reduced orotate phosphoribosyltransferase (encoded by pyrE) activity was isolated that exhibited a pyrimidine requirement for uracil or cytosine. Uptake of the nucleosides uridine or cytidine by wild-type or mutant cells was not detectable; explaining the inability of the mutant strain to utilize either nucleoside to satisfy its pyrimidine requirement. When the wildtype strain was grown in the presence of uracil, the activities of the five de novo enzymes were depressed. Pyrimidine limitation of the mutant strain led to the increase in aspartate transcarbamoylase and dihydroorotate dehydrogenase activities by more than 3-fold, and dihydroorotase and orotidine 5′-monophosphate decarboxylase activities about 1.5-fold, as compared to growth with excess uracil. It appeared that the syntheses of the de novo enzymes were regulated by pyrimidines. In vitro regulation of aspartate transcarbamoylase activity in P. pseudoalcaligenes ATCC 17440 was investigated using saturating substrate concentrations; transcarbamoylase activity was inhibited by Pi, PPi, uridine ribonucleotides, ADP, ATP, GDP, GTP, CDP, and CTP.
Similar content being viewed by others
References
Adair LB, Jones ME (1972) Purification and characteristics of aspartate transcarbamoylase from Pseudomonas fluorescens. J Biol Chem 247:2308–2315
Bachmann BJ (1972) Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev 36:525–557
Beckwith JR, Pardee AB, Austrian R, Jacob F (1962) Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol 5:618–634
Bergh ST, Evans DR (1993) Subunit structure of a class A aspartate transcarbamoylase from Pseudomonas fluorescens. Proc Natl Acad Sci USA 90:9818–9822
Borchardt S, Jayaswal RK (1993) Characterization of Tn5-259-induced pyrimidine mutants of Pseudomonas cepacia. Curr Microbiol 26:257–259
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye-binding. Anal Biochem 72:248–254
Chu C-P, West TP (1990) Pyrimidine biosynthetic pathway of Pseudomonas fluorescens. J Gen Microbiol 136:875–880
Condon S, Collins JK, O'Donovan GA (1976) Regulation of arginine and pyrimidine biosynthesis in Pseudomonas putida. J Gen Microbiol 92:375–383
Isaac JH, Holloway BW (1968) Control of pyrimidine biosynthesis in Pseudomonas aeruginosa. J Bacteriol 96:1732–1741
Matsumoto H, Nakazawa T, Ohta S, Terawaki Y (1981) Chromosomal locations of catA, pobA, pcaA, dcu and chu genes in Pseudomonas aeruginosa. Genet Res Camb 38:251–266
Munch-Petersen A, Mygind B (1976) Nucleoside transport systems in Escherichia coli K12: specificity and regulation. J Cell Physiol 89:551–560
Neumann J, Jones ME (1964) End-product inhibition of aspartate transcarbamylase in various species. Arch Biochem Biophys 104:438–447
O'Donovan GA, Neuhard J (1970) Pyrimidine metabolism in microorganisms. Bacteriol Rev 34:278–343
Prescott LM, Jones ME (1969) Modified methods for the determination of carbamyl aspartate. Anal Biochem 32:408–419
Ralston-Barrett E, Palleroni NJ, Doudoroff M (1976) Phenotypic characterization and deoxyribonucleic acid homologies of the ‘Pseudomonas alcaligenes’ group. Int J Syst Bacteriol 26:421–426
Stanier RY, Palleroni NJ, Doudoroff M (1966) The aerobic pseudomonads: a taxonomic study. J Gen Microbiol 43:159–271
Watson JM, Holloway BW (1976) Suppressor mutations in Pseudomonas aeruginosa. J Bacteriol 125:780–786
West TP (1989) Isolation and characterization of thymidylate synthetase mutants of Xanthomonas maltophilia. Arch Microbiol 151:220–222
West TP (1991) Pyrimidine base and ribonucleoside utilization by the Pseudomonas alcaligenes group. Antonie Van Leeuwenhoek 59:263–268
West TP, Chu C (1990) Regulation of pyrimidine biosynthesis in Pseudomonas cepacia. Arch Microbiol 154:407–409
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
West, T.P. Control of the pyrimidine biosynthetic pathway in Pseudomonas pseudoalcaligenes . Arch. Microbiol. 162, 75–79 (1994). https://doi.org/10.1007/BF00264376
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00264376