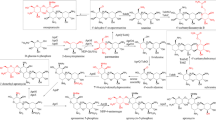
Abstract
Production of the macrolide antibiotic tylosin byStreptomyces T59-235 was inhibited in cultures containing high phosphate concentrations (30 mM Pi). Vegetative growth (dry weight increase, DNA and RNA synthesis) was hardly affected. Tylosin production began when macromolecule synthesis had slowed down to minimum level; in cultures with 30 mM Pi the onset of antibiotic production was retarded compared to cultures with low phosphate concentration (5 mM). The activities of three enzyme systems involved in tylosin biosynthesis (dTDP-D-glucose-4,6-dehydratase; dTDP-mycarose-forming enzyme system; SAM: macrocin-O-methyl transferase) were measured and found to be significantly lower in cultures with 30 mM Pi than in low phosphate cultures.
Chloramphenicol, but not rifampicin, caused a rapid decrease of both tylosin formation rate and dTDP-D-glucose-4,6-dehydratase activity when added to tylosin producing cultures.
Similar content being viewed by others
Abbreviations
- Pi :
-
inorganic phosphate
- dTDB:
-
2′-deoxythymidine diphosphate
- SAM:
-
S-adenosyl-L-methionine
- LP:
-
low phosphate (5 mM)
- HP:
-
high phosphate (30 mM)
References
Grisebach H (1978) Biosynthesis of sugar components of antibiotic substances. Adv Carbohydr Chem Biochem 35:81–126
Hostalek Z, Behal V, Curdova E, Jechova V (1979) Specific primary pathways supplying secondary biosynthesis. In: Genetics of industrial microorganisms OK Sebek, AJ Laskin (eds), Washington D.C.: American Society for Microbiology, pp 225–232
Liras P, Villanueva JR, Martin JF (1977) Sequential expression of macromolecule biosynthesis and candicidin formation inStreptomyces griseus. J Gen Microbiol 102:269–277
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275
Madry N, Pape H (1981) Regulation of tylosin biosynthesis by phosphate — possible involvement of transcriptional control. In: Actinomycetes, Zbl Bakt Supp 11 (KP Schaal, G Pulverer (eds). Fischer, Stuttgart New York, pp 441–445
Madry N, Sprinkmeyer R, Pape H (1979) Regulation of tylosin synthesis inStreptomyces: Effects of glucose analogs and inorganic phosphate. Eur J Appl Microbiol Biotechnol 7:365–370
Martin, JF (1977) Control of antibiotic synthesis by phosphate. Adv Biochem Eng 6:105–127
Martin JF, Demain AL (1980) Control of antibiotic synthesis. Microbiol Rev 44:230–251
Martin JF, Liras P, Demain AL (1977) Inhibition by phosphate of the activity of candicidin synthesis. FEMS Microbiol Lett 2:173–176
Martin JF, Gil JA, Naharro G, Liras P, Villanueva JR (1979) Industrial microorganisms tailor-made by removal of regulatory mechanisms. In: OK Sebek, AI Laskin (eds) Genetics of industrial microorganisms. Washington D.C.: American Society for Microbiology, pp 205–209
Matern H, Brillinger GU, Pape H (1973) Stoffwechselprodukte von Mikroorganismen. 114. Mitt. Thymidin-diphospho-D-glucoseoxido-reductase ausStreptomyces rimosus. Arch Mikrobiol 88:37–48
Morin RB, Gorman M, Hamill RL, Demarco PV (1970) The structure of tylosin. Tetrahedron Lett 54:4737–4740
Pape H, Brillinger GU (1973) Stoffwechselprodukte von Mikroorganismen. 113. Mitt. Biosynthes von Thymidin-diphosphomycarose durch ein zellfreies System ausStreptomyces rimosus. Arch Mikrobiol 88:25–35
Seno ET, Pieper RL, Huber FM (1977) Terminal stages in the biosynthesis of tylosin. Antimicrob. Agents Chemother 11:455–461
Simuth J, Hudec J, Chan HT, Danyi O, Zelinka J (1979) The synthesis of highly phosphorylated nucleotides, RNA and protein byStreptomyces aureofaciens. J Antibiot 32:53–58
Sprinkmeyer R, Pape H (1978) Effects of glucose and fatty acids on the formation of the macrolide antibiotic tylosin byStreptomyces. In: Genetics of the actinomycetales, E Freerksen, I Tarnok, JH Thumin (eds). Fischer, Stuttgart New York, pp 51–58
Vu-Trong K, Bhuwapathanapun, S, Gray PP (1980) Metabolic regulation in tylosin-producingStreptomyces fradiae: Regulatory role of adenylate nucleotide pool and enzymes involved in biosynthesis of tylonolide precursors. Antimicrob Agents Chemother 17:519–525
Weinberg, ED (1974) Secondary metabolism: Control by temperature and inorganic phosphate. Develop Ind Microbiol 15:70–81
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madry, N., Pape, H. Formation of secondary metabolism enzymes in the tylosin producerStreptomyces T59-235. Arch. Microbiol. 131, 170–173 (1982). https://doi.org/10.1007/BF01054001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01054001