Abstract
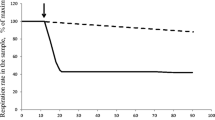
In chemostat cultures of Bacillus caldolyticus, adaptation in a single step from 70–100°C was followed under aerobic and oxygen-limited conditions and was found to proceed more smoothly under the latter circumstances. Variations of the medium (e.g. yeast extract or silicate concentrations) showed that growth at 100°C is in all respects similar to that of cultures at moderate temperatures.
Enzyme preparations derived from cultures at 5°C intervals between 70 and 100°C were used to determine the temperature range. For all nine enzymes tested, the optimum temperature was found to be 67°C; the latter was independent of the growth temperature. Differences were found, however, with respect to the maximum temperature of individual enzymes, and three groups, with maxima between 70 and 80°C, 80 and 90°C and 90 and 100°C can be distinguished. Again, there was no correlation with the growth temperature.
Stability experiments also revealed that enzymes from the same organism can have different thermal properties: Some were found to be quite thermolabile (e.g. the pyruvate kinase), while others (e.g. hexokinase and glutamate-pyruvate transaminase) exhibited a high thermostability. These properties were not related to the growth temperature within the 70–100°C range, too.
Six of the enzymes tested could be stabilized by their respective substrates, but the degree of protection varied for individual enzymes. Three enzymes (acetate kinase, glutamate dehydrogenase and myokinase) could not be stabilized by their substrates.
Comparative experiments with the hexokinase suggested, that the thermal integrity of the enzymes is better protected within the cell as compared to the stability of the enzyme preparations.
Similar content being viewed by others
Abbreviations
- AK:
-
acetate kinase
- Ala-DH:
-
alanine dehydrogenase
- Ald:
-
aldolase
- GIDH:
-
glutamate dehydrogenase
- G6P-DH:
-
glucose-6-phosphate dehydrogenase
- GTP:
-
glutamate-pyruvate transaminase
- HK:
-
hexokinase
- ICDH:
-
isocitrate dehydrogenase
- MK:
-
myokinase
- PK:
-
pyruvate kinase
References
Bergmeyer HK (1970) Methoden der enzymatischen Analyse, 2. Aufl., Verlag Chemie, Weinheim/Bergstr., Bd. I pp 390–719, BdII pp 1062–1623
Grootegoed JA, Lauwers AM, Heinen W (1973) Separation and partial purifications of extracellular amylase and protease from Bacillus caldolyticus. Arch Mikrobiol 90:223–232
Gunter BD (1968) Geochemical and isotopic studies of hydrothermal gases and waters. University Microfilms, Inc., Ann Arbor, Michigan, 68-9658. Ph. D. Thesis
Haberstich HU, Zuber H (1974) Thermoadaptation of enzymes in thermophilic and mesophilic cultures of Bacillus stearothermophilus and Bacillus caldotenax. Arch Microbiol 98:275–287
Heinen UJ, Heinen W (1972) Characteristics and properties of a caldoactive bacterium producing extracellular enzymes and two related strains. Arch Mikrobiol 82:1–23
Heinen W, Lauwers AM (1975) Variability of the molecular size of extracellular amylase produced by intact cells and protoplasts of Bacillus caldolyticus. Arch Microbiol 106:201–207
Heinen W, Lauwers AM (1976) Amylase activity and stability at high and low temperature depending on calcium and other divalent cations. In: Zuber H (ed) Enzymes and proteins from thermophilic microorganisms. Birkhäuser, Basel Stuttgart, pp 77–89
Heinen W, Lauwers AM (1981) Growth of bacteria at 100°C and beyond. Arch Microbiol 129:127–128
Hengartner H, Zuber H (1973) Isolation and characterization of a thermophilic glucokinase from Bacillus stearothermophilus. FEBS Letters 37:212–216
Kitano Y (ed) (1975) Geochemistry of water. Dowden, Hutchinson and Rosa, Inc. Stroudsburg, PA, pp 278–279
Wedler FC, Hoffmann FM, Kenny R, Carfi J (1976) Maintainance of specificity information and thermostability in thermophilic Bacillus sp. glutamine synthetase. In: Zuber H (ed) Enzymes and proteins from thermophilic microorganisms. Birkhäuser, Basel Stuttgart, pp 187–197
Yutani K, Ogasahara K, Sugino Y, Matsushiri A (1977) Effect of a single amino acid substitution on stability of conformation of a protein Nature 267:274–275
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lauwers, A.M., Heinen, W. & Mulders, J.W.M. Properties of enzymes from bacteria grown in the 70–100°C range. Arch. Microbiol. 130, 159–164 (1981). https://doi.org/10.1007/BF00411071
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00411071