Abstract
Photosynthesis was measured by the 14C method on natural as well as low light adapted populations of Chloroflexus (a photosynthetic bacterium) and Synechococcus (a blue-green alga) from hot springs in Yellowstone National Park (Wyoming U.S.A.), to test the ability of these phototrophs to photosynthesize at a variety of light intensities. The herbicide 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (DCMU) was used to distinguish uptake of the blue-green alga from that of the photosynthetic bacterium, while measurements of chlorophyll a and bacterio-chlorophyll c served to quantitate the standing crops of these organisms.
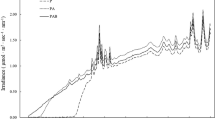
Natural populations of Synechococcus were found to be slightly inhibited by full sunlight intensities (summer values can surpass 90000 Lux), whereas the Chloroflexus populations were not. Populations of both phototrophs subjected to reduced light intensities through the use of neutral density filters were found to adapt to low light, and then become severely inhibited by high light intensities. Adaptation to various light regimes may be an important ecological phenomenon to the survival of these hot spring phototrophs.
Similar content being viewed by others
References
Bauld, J., Brock, T. D.: Ecological studies of Chloroflexis, a gliding photosynthetic bacterium. Arch. Mikrobiol. 92, 267–284 (1973)
Bott, T. L., Brock, T. D.: Bacterial growth rates above 90°C in Yellowstone hot springs Science 164, 1411–1412 (1969)
Brock, T. D., Brock, M. L.: Temperature optima for algal development in Yellowstone and Iceland hot springs. Nature (Lond.) 209, 733–734 (1966)
Brock, T. D., Brock, M. L.: Effect of light intensity on photosynthesis by thermal algae adapted to natural and reduced sunlight. Limnol. Oceanog. 14, 334–341 (1969)
Cohen-Bazire, G., Sistrom, W. R.: The procaryotic photosynthetic apparatus. In: The chlorophylls (L. P. Vernon, G. R. Seely, eds.), pp. 313–341. New York: Academic Press 1966
Doemel, W. N., Brock, T. D.: Bacterial stromatolites: Origin of laminations. Science 184 1083–1085 (1974)
Doemel, W. N., Brock, T. D.: Vertical distribution of sulfur species in benthic algal mats. Limnol. Oceanog. 21, 237–244 (1976)
Gates, D. M.: Energy exchange in the biosphere. New York Harper and Row 1962
Goldman, D. R., Mason, D. T., Wood, B. J. B.: Light injury and inhibition in antarctic freshwater phytoplankton. Limnol. Oceanog 8, 313–322 (1963)
Halfen, L. N., Pierson, B. K., Francis, G. W.: Carotenoids of a gliding organism, containing bacteriochlorophylls. Arch. Mikrobiol. 82, 240–246 (1972)
Holt, S. C., Marr, A. G.: Effect of light intensity on the formation of intracytoplasmic membrane in Rhodospirillum rubrum. J. Bact. 89, 1421–1429 (1965)
Holt, S. C., Conti, S. F., Fuller, R. C.: Effect of light intensity on the formation of the photochemical apparatus in the green bacterium Chloropseudomonas ethylicum. J. Bact. 91, 349–355 (1966)
Kimball, H. H.: Records of total solar radiation intensity and their relation to daylight intensity. Monthly Weather Rev. 52, 473–479 (1924)
Kok, B.: Oh inhibition of photosynthesis by intense light. Biochim. biophys. Acta (Amst.) 21, 234–244 (1956)
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951)
Madigan, M. T., Brock, T. D.: Quantitative estimation of bacteriochlorophyll c in the presence of chlorophyll a in aquatic environments Limnol. Oceanog. 21, 462–467 (1976)
Pfennig, N.: Photosynthetic bacteria. Ann. Rev. Microb. 21, 285–324 (1967)
Pierson, B. K., Castenholz, R. W.: Bacteriochlorophylls in gliding filamentous procaryotes from hot springs. Nature New Biol 233 25–27 (1971)
Pierson, B. K., Castenholz, R. W.: A phototrophic gliding filamentous bacterium in hot springs. Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 100, 5–24 (1974a)
Pierson, B. K., Castenholz, R. W.: Studies of pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium. Arch. Microbiol. 100, 283–305 (1974b)
Shiokawa, K., Takahashi, M., Ichimura, S.: Physiological adaptation of photosynthetic bacteria to low light and its ecological meaning. Jap. J. Limnol. 34, 1–11 (1973)
Sorokin, Y. I.: Interrelations between sulphur and carbon turnover in meromictic lakes. Arch Hydrobiol. 66, 391–446 (1970)
Trentini, W. C., Starr, M. P.: Growth and ultrastructure of Rhodomicrobium vannielii as a function of light intensity. J. Bact. 93, 1699–1704 (1967)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madigan, M.T., Brock, T.D. Adaptation by hot spring phototrophs to reduced light intensities. Arch. Microbiol. 113, 111–120 (1977). https://doi.org/10.1007/BF00428590
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00428590