Abstract
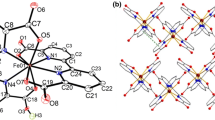
The X-ray crystal structure of trinuclear iron acetate [Fe3O(CH3COO)6(H2O)3]2 [ZnCl4] ⋅ 2H2O was determined. The crystal has a ionic structure. It is monoclinic, a = 25.363(7), b = 14.533(4), c = 15.692(4) Å, β = 103.11(2)°, space group C2/c, and R = 0.0685. The structure of the cationic complex [Fe3O(CH3COO)6(H2O)3]+ is typical of trinuclear iron(III) compounds containing a μ3-O bridge: the iron atoms are situated at the vertices of an almost equilateral triangle with the O atom at the center. Each Fe atom is coordinated by four O atoms of bridging carboxy groups, the μ3-bridging O atom, and the water molecule in the trans position to the latter O atom. Mössbauer spectroscopy evidence indicates the high-spin state (S = 5/2) of the iron(III) ions.
Similar content being viewed by others
References
M. A. Porai-Koshits, Itogi Nauki Tekh., Ser.: Kristallokhim. 15, 3 (1981).
B. O. West, Polyhedron 4, 219 (1989).
E. L. Muettertis, Catal. Rev. 23(2), 69 (1981).
R. C. Mehrota and R. Bohra, Metal Carboxylates (Academic Press, London, 1983).
R. D. Cannon and R. P. White, Progr. Inorg. Chem. 36, 196 (1988).
S. I. Lippard, Angew. Chem. Int. Ed. Engl. 27, 344 (1988).
B. Vincent, G. L. Oliver-Lilley, and B. A. Averill, Chem. Rev. 90(8), 1447 (1990).
R. R. Crichton, Inorganic Biochemistry of Iron Metabolism (Harvard, New York, 1991).
K. Anzenhofer and J. J. de Boer, Rec. Trav. Chim. Pays-Bas 88(3), 286 (1969).
K. Wieghardt, K. Pohl, I. Jibril, and C. Huttner, Angew. Chem. 96, 66 (1984).
K. L. Taft, G. C. Papaefthymiou, and S. J. Lippard, Science 259, 1302 (1993).
W. Micklitz and S. J. Lippard, J. Am. Chem. Soc. 111(17), 6856 (1989).
A. K. Powell, S. L. Heath, and D. Gatteschi, J. Am. Chem. Soc. 117(9), 2491 (1995).
C. I. Turta, S. G. Shova, and F. A. Spatar’, Zh. Strukt. Khim. 35(2), 112 (1994).
T. Glowiak, M. Kubiak, T. Szymanska-Buzar, and B. Jezowska-Tyzebiatowska, Acta Crystallogr., Sect. B 33, 3106 (1977).
S. Shova, C. Turta, I. Cadelńic, and V. Meriacre, in Proceedings of the Conference on Chemistry and Chemical Technology, Bucharest, Romania, 1997 (Bucharest, 1997), Vol. 1, p. 1.
S. G. Shova, I. G. Cadelńic, and M. Gdaniec, Zh. Strukt. Khim. 39(5), 917 (1999).
I. Cadelńic, S. Shova, and Yu. A. Simonov, Pol. J. Chem. 71, 501 (1997).
G. M. Sheldrick, Acta Crystallogr., Sect. A 46, 467 (1990).
G. M. Sheldrick, SHELXL93: Program for the Refinement of Crystal Structures (Univ. of Göttingen, Göttingen, 1993).
C. E. Anson, J. P. Bourke, and R. D. Cannon, Inorg. Chem. 36, 1265 (1997).
V. M. Lynch, J. M. Sibert, J. L. Sessler, and B. E. Davis, Acta Crystallogr., Sect. C 47(4), 866 (1991).
T. Sato and F. Ambe, Acta Crystallogr., Sect. C 52, 3005 (1996).
S. G. Shova, I. G. Cadelńic, and T. C. Jovmir, Koord. Khim. 23(9), 672 (1997) [J. Coord. Chem. 23, 629 (1997)].
C. I. Turta, A. G. Lézéresku, and Yu. A. Simonov, Koord. Khim. 22(1), 45 (1996) [J. Coord. Chem. 22, 42 (1996)].
F. Degang, W. Guoxiong, T. Wenxia, and Y. Kaibei, Polyhedron 12(20), 2459 (1993).
Chemical Applications of Mössbauer Spectroscopy, Ed. by V. I. Goldanskii and R. H. Herber (Academic Press, New York, 1968; Mir, Moscow, 1970).
R. V. Pound and G. A. Rebka, Phys. Rev. Lett. 4(5–6), 274 (1960).
F. A. Spatar’, A. T. Lézéresku, and C. I. Turta, Zh. Obshch. Khim. 66(1), 12 (1996).
F. A. Spatar’, V. M. Meriakre, and V. E. Zubareva, Koord. Khim. 22(3), 188 (1996) [J. Coord. Chem. 22, 176 (1996)].
G. Filotti, V. Meriacre, and A. Avramescu, in Proceedings of ICAME-95 (Rimini, Italy, 1995), O1–D9.
Author information
Authors and Affiliations
Additional information
__________
Translated from Kristallografiya, Vol. 45, No. 3, 2000, pp. 458–464.
Original Russian Text Copyright © 2000 by Shova, Cadelnic, Gdaniec, Simonov, Jovmir, Filoti, Bulhac, Turta.
Rights and permissions
About this article
Cite this article
Shova, S.G., Cadelnic, I.G., Gdaniec, M. et al. Crystal and molecular structure of complex compound [Fe3O(CH3COO)6(H2O)3]2[ZnCl4] ⋅ 2H2O. Crystallogr. Rep. 45, 416–421 (2000). https://doi.org/10.1134/1.171209
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/1.171209