Abstract
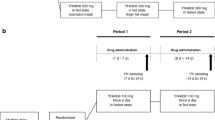
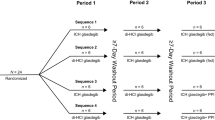
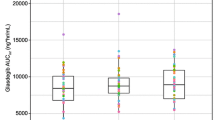
The absorption of three combination formulations of hydrochlorothiazide and either triamterene or amiloride was studied over a 5-year period in seven separate investigations under varying conditions of food and fasting. The most widely prescribed combination, containing 25 mg of hydrochlorothiazide and 50 mg of triamterene, demonstrated impaired absorption in the fasting state that was partially corrected by the addition of a breakfast high in fat. The increase in the fat content of the food appeared to correlate directly with the amount of both drugs absorbed from this formulation. The second formulation studied, a new combination formulation of 50 mg of hydrochlorothiazide and 75 mg of triamterene, demonstrated acceptable absorption in the fasting state that was not altered by the concurrent administration of a high-fat breakfast. The absorption of the third formulation, a combination of 50 mg hydrochlorothiazide and 5 mg amiloride, was acceptable in the fasting state and demonstrated a slight reduction in the absorption of the amiloride component when administered concurrently with a high-fat meal. The clinical and biopharmaceutic implications of these observations are discussed.
Similar content being viewed by others
REFERENCES
R. A. Upton, R. L. Williams, E. T. Lin, W. L. Gee, C. D. Blume, and L. Z. Benet. J. Pharmacokinet. Biopharm. 12:575–586 (1984).
C. D. Blume, R. L. Williams, R. A. Upton, E. T. Lin, and L. Z. Benet. Am. J. Med. 77 (Suppl 5A):59–61 (1984).
Metropolitan Life Insurance Company Health and Safety Education Division Bulletin, (1959 or) 1983.
Food and Drug Administration. Food and Drug Administration Guidelines for the Evaluation of Controlled Release Drug Products, May 1984.
E. T. Lin. In P. M. Kabra and L. J. Martion (eds.), Clinical Liquid Chromatography, Vol. 1, CRC Press, Boca Raton, Fla., 1985, pp. 115–118.
E. T. Lin. In P. M. Kabra and L. J. Martion (eds.), Clinical Liquid Chromatography, Vol. 1, CRC Press, Boca Raton, Fla., 1985, pp. 123–127.
R. J-y. Shi, L. Z. Benet, and E. T. Lin. J. Chromatogr. 377 (1986) (in press).
H. Minami and R. W. McCallum. Gastroenterology 86:1592–1610 (1984).
C. J. Carr. Annu. Rev. Pharmacol. Toxicol. 22:19–29 (1982).
P. G. Welling. Clin. Pharmacokinet. 9:404–434 (1984).
P. G. Welling. L. L. Lyons, W. A. Craig, and G. A. Trochta. Clin. Pharm. Ther. 17:475–480 (1975).
G. Heimann, J. Murgescu, and U. Bergt. Eur. J. Clin. Pharmacol. 22:171–173 (1982).
N. H. Leeds, P. Gal, A. A. Purohit, and J. B. Walter. J. Clin. Pharmacol. 22:196–200 (1982).
M. A. Osman, R. B. Patel, D. S. Irwin, and P. G. Welling. Biopharm. Drug Disp. 4:63–72 (1983).
A. Karim. Amer. Pharm. NS25:132–142 (1985).
A. Karim, T. Burns, L. Wearley, J. Streicher, and M. Palmer. Clin. Pharm. Ther. 38:77–83 (1985).
A. Karim, T. Burns, D. Janky, and A. Hurwitz. Clin. Pharm. Ther. 38:624–647 (1985).
C. K. Svensson, D. J. Edwards, P. M. Mauriello, S. H. Barde, A. C. Foster, R. A. Lanc, E. Middleton, Jr., and D. Lalka. Clin. Pharm. Ther. 34:316–323 (1983).
A. J. McLean, P. J. McNamara, P. duSouich, M. Gibaldi, and D. Lalka. Clin. Pharm. Ther. 24:5–10 (1978).
A. Melander and A. McLean. Clin. Pharmacokinet. 8:286–296 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williams, R.L., Mordenti, J., Upton, R.A. et al. Effects of Formulation and Food on the Absorption of Hydrochlorothiazide and Triamterene or Amiloride from Combination Diuretic Products. Pharm Res 4, 348–352 (1987). https://doi.org/10.1023/A:1016409606936
Issue Date:
DOI: https://doi.org/10.1023/A:1016409606936